Annual Report 2017
Cancer Screening Center
Takahisa Matsuda, Masau Sekiguchi, Keiko Nakamura, Yasuo Kakugawa, Minori Matsumoto, Masayoshi Yamada, Hiroyuki Takamaru, Takaaki Tsuchida, Hidetsugu Yamagishi, Yasuaki Arai, Gen Iinuma, Nachiko Uchiyama, Hiroaki Kurihara, Miyuki Sone, Yasunori Mizuguchi, Hirokazu Watanabe, Mari Kikuchi, Mototaka Miyake, Syunsuke Sugawara, Yuko Kubo, Takahiro Morita, Koji Tomita, Shinji Wada, Tomoyasu Kato, Shunichi Ikeda, Mitsuya Ishikawa, Takashi Uehara, Hanako Shimizu, Koichi Nagata, Hiroshi Saito, Mika Mori, Ryutaro Kakinuma (Visiting Researcher)
Introduction
In the Cancer Screening Center (former name: Research Center for Cancer Prevention and Screening: RCCPS), we provide opportunistic cancer screening widely by using newly developed modalities since 2004. Most of the staff doctors hold two positions concurrently with in both the Cancer Screening Center and their own specialized departments. Our Cancer Screening Center consists of 14 radiologists, nine gastroenterologists, one bronchoscopist, five gynecologists, seven radiologic technologists, five ultrasonographic technologists, and four nurses. We are in charge of multiphasic cancer screening using several imaging modalities to develop new cancer screening systems and to assess new screening tests. All medical images are digitalized and all imaging diagnosis can be made from CRT monitors.
Our team and what we do
1. Course of cancer screening
The basic plan for males consists of the screening for cancers of the lung, esophagus, stomach, colorectum, liver, gall bladder, pancreas, kidney, and prostate. In the basic plan for females, the screening for cancers of the breast, uterus, and ovary are added to the plan for males, excluding the prostate. In addition, Positron Emission Tomography (PET) is provided as an option. Other than multiphasic programs, an independent cancer screening program has been prepared for lung and female genital cancers, including cancers of the uterus and ovary, breast cancer, and gastrointestinal cancer. Blood samples are also obtained for biochemistry and tumor markers such as CA19-9, CEA, CA125, PSA, and genetic analysis.
2. Eligibility criteria for participants
The cancer screening program at the Cancer Screening Center (former name: RCCPS) before 2013 has been planned for applicants 40 years or older who give written informed consent for the screening, including blood samples for genetic analysis, and who take the questionnaire survey concerning lifestyles. These study protocols have been approved by the Institutional Review Board (IRB). Applicants who have been diagnosed as having cancer, and/or have a history of cancer treatment, such as surgery or endoscopic mucosal resection or chemotherapy within the previous one year, are excluded. In contrast, there is no condition setting to receive cancer screening programs about new participants after May, 2014. But an inclusion agreement about the study is optionally demanded.
3. Cancer screening methods
In multiphasic cancer screening programs, computed tomography (CT) for lung cancer, abdominal ultrasound (US) for cancer of the liver, gall bladder, pancreas, and kidney, gynecological examinations with pap-smear and human papillomavirus (HPV) test for uterus cancer, and Mammography (MMG)/ Tomosynthesis and US for breast cancer are performed on the first day. On the following day, gastroscopy for cancer of the esophagus and stomach, and total colonoscopy for cancer of the colon and rectum are conducted. If a barium enema is chosen, the examination is carried out on the third day. Moreover, from the beginning of December 2010, CT-colonography (CTC) has been provided as an optional method for cancer screening. Fluorodexyglucose (FDG)-PET is offered on the first day as an option, if participants wish to undergo the examination. In addition, the one day cancer screening program with the combination of gastrointestinal endoscopic examinations and other methods except PET, or the combination of PET and other methods excepting total colonoscopy were newly started in May, 2014. Furthermore, methionine PET-CT/ Magnetic Resonance Imaging (MRI) has been provided as an optional examination since 2016.
4. Number of participants of cancer screening
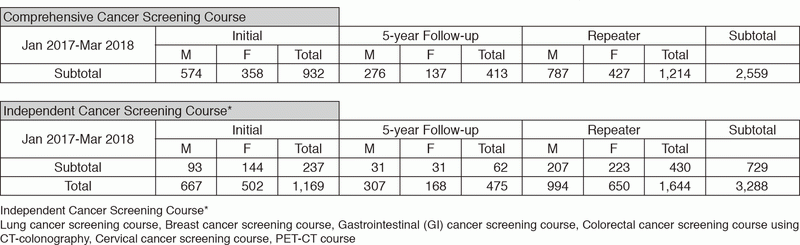
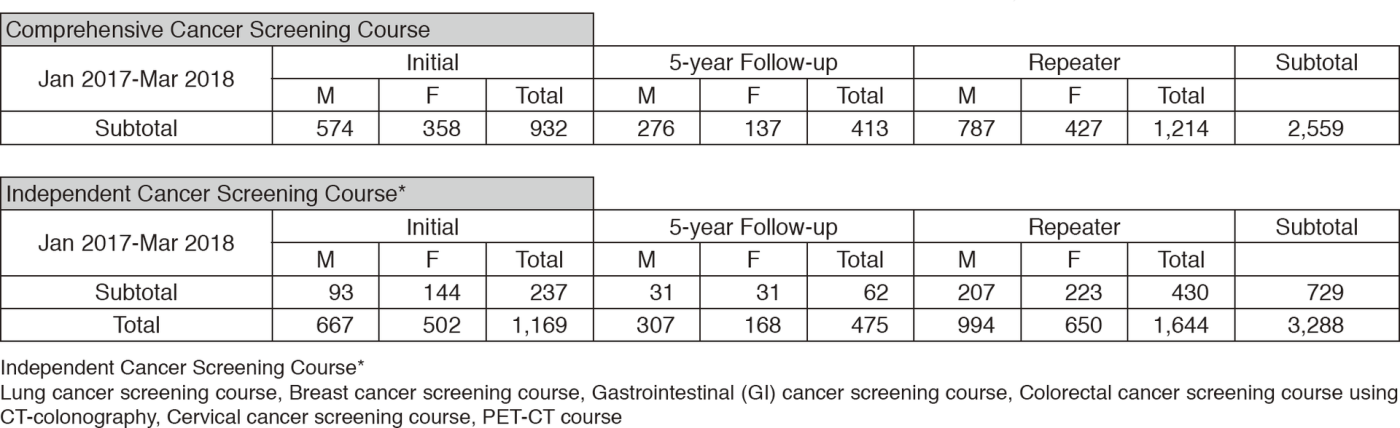
We present the number of participants of cancer screening during the period from January
2017 to March 2018 in this report (Table 1). A
total of 3,288 people including 1,169 initial cases received cancer screening at the Cancer Screening Center in this period. Most of the participants (78%; n=2,559) chose the comprehensive cancer screening course. Regarding the data of cancer detection rate in each modality, we will report them in the near future.
Research activities
We published three English articles during this period on the results of the examinees who underwent cancer screening at our center as follows.
1) A large-scale analysis of diagnostic sensitivity of PET-CT for esophageal cancer
2) Association of plasma C-reactive protein level with the prevalence of colorectal adenoma
3) A scoring model for predicting advanced colorectal neoplasia in a screened population of asymptomatic Japanese individuals
Clinical trials
In our center, we are conducting research work based on the study protocol titled "Evaluation of effectiveness of cancer screening modality at National Cancer Center". The target modalities are as follows.
1) Upper gastrointestinal endoscopy
2) Lower gastrointestinal endoscopy
3) CT colonography
4) Chest computed tomography (CT)
5) Sputum cytology
6) Mammography
7) Breast ultrasonography
8) FDG-positron emission tomography (PET)
9) Abdominal ultrasonography
10) Serum tumor markers
Future prospects
On the basis of cancer screening data such as examination results, medical institution findings, follow-up findings, and the questionnaire survey concerning lifestyles for 10 years, we commenced to assess them supported by the National Cancer Center Research and Development Fund.
List of papers published in January 2017 - March 2018
Journal
1. Nakamura F, Saito Y, Haruyama S, Sekiguchi M, Yamada M, Sakamoto T, Nakajima T, Yamamoto S, Murakami Y, Ishikawa H, Matsuda T. Short-term Prospective Questionnaire Study of Early Postoperative Quality of Life After Colorectal Endoscopic Submucosal Dissection. Dig Dis Sci, 62:3325-3335, 2017
2. Saito Y, Bhatt A, Matsuda T. Colorectal endoscopic submucosal dissection and its journey to the West. Gastrointest Endosc, 86:90-92, 2017
3. Sekiguchi M, Matsuda T, Saito Y. What is the optimal colorectal cancer screening program for an average-risk population? Transl Gastroenterol Hepatol, 2:17, 2017
4. Sekiguchi M, Terauchi T, Kakugawa Y, Shimada N, Saito Y, Matsuda T. Performance of 18-fluoro-2-deoxyglucose positron emission tomography for esophageal cancer screening. World J Gastroenterol, 23:2743-2749, 2017
5. Tamai N, Saito Y, Sakamoto T, Nakajima T, Matsuda T, Sumiyama K, Tajiri H, Koyama R, Kido S. Effectiveness of computer-aided diagnosis of colorectal lesions using novel software for magnifying narrow-band imaging: a pilot study. Endosc Int Open, 5:E690-E694, 2017
6. Sekiguchi M, Oda I. High miss rate for gastric superficial cancers at endoscopy: what is necessary for gastric cancer screening and surveillance using endoscopy? Endosc Int Open, 5:E727-E728, 2017
7. Oka S, Uraoka T, Tamai N, Ikematsu H, Chino A, Okamoto K, Takeuchi Y, Imai K, Ohata K, Shiga H, Raftopoulos S, Lee BI, Matsuda T. Standardization of endoscopic resection for colorectal tumors larger than 10 mm in diameter. Dig Endosc, 29 Suppl 2:40-44, 2017
8. Sekiguchi M, Oda I, Suzuki H, Abe S, Nonaka S, Yoshinaga S, Taniguchi H, Sekine S, Saito Y. Clinical outcomes and prognostic factors in gastric cancer patients aged >/=85 years undergoing endoscopic submucosal dissection. Gastrointest Endosc, 85:963-972, 2017
9. Matsuda T, Ono A, Sekiguchi M, Fujii T, Saito Y. Advances in image enhancement in colonoscopy for detection of adenomas. Nat Rev Gastroenterol Hepatol, 14:305-314, 2017
10. Yamada M, Saito Y, Takamaru H, Sasaki H, Yokota T, Matsuyama Y, Sato Y, Sakamoto T, Nakajima T, Taniguchi H, Sekine S, Matsuda T. Long-term clinical outcomes of endoscopic submucosal dissection for colorectal neoplasms in 423 cases: a retrospective study. Endoscopy, 49:233-242, 2017
11. Hotta K, Matsuda T, Kakugawa Y, Ikematsu H, Kobayashi N, Kushima R, Hozawa A, Nakajima T, Sakamoto T, Mori M, Fujii T, Saito Y. Regional colorectal cancer screening program using colonoscopy on an island: a prospective Nii-jima study. Jpn J Clin Oncol, 47:118-122, 2017
12. Suzuki H, Oda I, Abe S, Sekiguchi M, Nonaka S, Yoshinaga S, Saito Y, Fukagawa T, Katai H. Clinical outcomes of early gastric cancer patients after noncurative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer, 20:679-689, 2017
13. Nakamura K, Nonaka S, Nakajima T, Yachida T, Abe S, Sakamoto T, Suzuki H, Yoshinaga S, Oda I, Matsuda T, Sekine S, Kanemitsu Y, Katai H, Saito Y, Hirota S. Clinical outcomes of gastric polyps and neoplasms in patients with familial adenomatous polyposis. Endosc Int Open, 5:E137-E145, 2017
14. Ikematsu H, Sakamoto T, Togashi K, Yoshida N, Hisabe T, Kiriyama S, Matsuda K, Hayashi Y, Matsuda T, Osera S, Kaneko K, Utano K, Naito Y, Ishihara H, Kato M, Yoshimura K, Ishikawa H, Yamamoto H, Saito Y. Detectability of colorectal neoplastic lesions using a novel endoscopic system with blue laser imaging: a multicenter randomized controlled trial. Gastrointest Endosc, 86:386-394, 2017
15. Ishihara R, Oyama T, Abe S, Takahashi H, Ono H, Fujisaki J, Kaise M, Goda K, Kawada K, Koike T, Takeuchi M, Matsuda R, Hirasawa D, Yamada M, Kodaira J, Tanaka M, Omae M, Matsui A, Kanesaka T, Takahashi A, Hirooka S, Saito M, Tsuji Y, Maeda Y, Yamashita H, Oda I, Tomita Y, Matsunaga T, Terai S, Ozawa S, Kawano T, Seto Y. Risk of metastasis in adenocarcinoma of the esophagus: a multicenter retrospective study in a Japanese population. J Gastroenterol, 52:800-808, 2017
16. Budhathoki S, Iwasaki M, Yamaji T, Yamamoto H, Kato Y, Tsugane S. Association of plasma concentrations of branched-chain amino acids with risk of colorectal adenoma in a large Japanese population. Ann Oncol, 28:818-823, 2017
17. Inoki K, Sakamoto T, Takamaru H, Sekiguchi M, Yamada M, Nakajima T, Matsuda T, Taniguchi H, Sekine S, Kanemitsu Y, Ohe Y, Saito Y. Predictive relevance of lymphovascular invasion in T1 colorectal cancer before endoscopic treatment. Endosc Int Open, 5:E1278-E1283, 2017
18. Cho H, Sekine S, Sekiguchi M. Adenocarcinoma of the colon presenting as a submucosal tumor. Dig Endosc, 30:114-115, 2018
19. Kodashima S, Tanaka K, Matsuda K, Fujishiro M, Saito Y, Ohtsuka K, Oda I, Katada C, Kato M, Kida M, Kobayashi K, Hoteya S, Horimatsu T, Matsuda T, Muto M, Yamamoto H, Ryozawa S, Iwakiri R, Kutsumi H, Miyata H, Haruma K, Fujimoto K, Uemura N, Kaminishi M, Tajiri H. First progress report on the Japan Endoscopy Database project. Dig Endosc, 30:20-28, 2018
20. Matsuda K, Tanaka K, Fujishiro M, Saito Y, Ohtsuka K, Oda I, Katada C, Kato M, Kida M, Kobayashi K, Hoteya S, Horimatsu T, Kodashima S, Matsuda T, Muto M, Yamamoto H, Ryozawa S, Iwakiri R, Kutsumi H, Miyata H, Haruma K, Fujimoto K, Uemura N, Kaminishi M, Tajiri H. Design paper: Japan Endoscopy Database (JED): A prospective, large database project related to gastroenterological endoscopy in Japan. Dig Endosc, 30:5-19, 2018
21. Abe S, Oda I, Minagawa T, Sekiguchi M, Nonaka S, Suzuki H, Yoshinaga S, Bhatt A, Saito Y. Metachronous Gastric Cancer Following Curative Endoscopic Resection of Early Gastric Cancer. Clin Endosc, 51:253-259, 2018
22. Sakamoto T, Saito Y, Nakamura F, Abe S, Takamaru H, Sekiguchi M, Yamada M, Nakajima T, Matsuda T, Yamagishi H, Kato H. Short-term outcomes following endoscopic submucosal dissection of large protruding colorectal neoplasms. Endoscopy, 50:606-612, 2018
23. Bogie RMM, Veldman MHJ, Snijders LARS, Winkens B, Kaltenbach T, Masclee AAM, Matsuda T, Rondagh EJA, Soetikno R, Tanaka S, Chiu HM, Sanduleanu-Dascalescu S. Correction: Endoscopic subtypes of colorectal laterally spreading tumors (LSTs) and the risk of submucosal invasion: a meta-analysis. Endoscopy, 50:C4, 2018
