Center for Research Administration and Support (CRAS)
Teruhiko Yoshida, Takeyuki Sato, Kiyohito Nakai, Nobuko Ushirozawa, Yutaka Inazu, Namiko Aoshima, Fumie Suzuki, Kiyoka Nagayama, Yuko Yamada, Jinko Sato, Yuichi Amanuma, Miki Ito, Yoshihiro Wakabayashi, Shunichi Magara, Ayako Yamashita, Shou Kobayashi, Masaki Yamamoto, Yuji Shinoda, Genta Ohno, Yukari Nakayama, Yuichi Nakamura, Hiromi Nakagawa, Ryu Aoyama, Chieko Yano, Ryoko Noda, Kazunori Aoki, Kazuhiko Aoyagi, Taro Shibata, Gakuto Ogawa, Yasue Kojima, Megumi Kubo, Aya Kuchiba, Junki Mizusawa, Masashi Wakabayashi, Shogo Nomura, Shimon Tashiro, Tsunakuni Ikka, Akiko Nakada, Mao Shikano, Mari Hirata, Junko Okuaki, Satoko Tabata, Motonori Kakei, Noriko Yamashita, Yayoi Ofuji, Kuniko Takahashi, Sachiyo Taniguchi, Harumi Maruno, Kaori Matsushita, Shizuka Yamamoto, Tomoko Ishida, Yuka Higashi, Yuko Nagasawa, Ayu Goto, Junko Horie, Norie Enomoto, Nanako Takatera, Akiko Tsuchida, Haruka Nakada, Yuri Kai
Introduction
The Center for Research Administration and Support (CRAS) was established on July 16, 2014. The starting members of the CRAS were approximately 160 staff, who together offer diverse functions and specialties, ranging from research fund administrations, alliance with private sectors, intellectual properties, clinical research coordinators and data managers, monitoring and audit, biostatistics support, offices for research ethics (IRB), to COI committees.
The background and the purpose of the crea-tion of the CRAS was explained by Dr. TomomitsuHotta, the President of the National CancerCenter (NCC), in the NCC News 2014 Vol.5
No.3 (in Japanese). Briefly, since its foundation in 1962, the NCC has added several new segments and organizations to evolve as a comprehensive cancer center. Because each segment needed its own research infrastructure, support activities in the NCC had become fragmented and scattered with the possibility of gaps and redundancies. Dr. Hotta asked the Strategic Planning Bureau and assembled the "NCC New Vision" in 2014, in which he proposed integration and communications of various research support functions in the NCC. The CRAS was created in response to the 2014 Vision.
In 2015, the NCC Hospital (NCCH) and the NCC Hospital East (NCCHE) were certified as the Core Clinical Research Hospital under the Medical Care Act in August and September, respectively.
It is then required that the support functions for the clinical research, especially those concerning clinical trials, need to be operated under the explicit responsibilities of a hospital director. As a result, the governance of the Research Coordination Division, Research Promotion Division and Regulatory Science Section of the CRAS has now moved to the Clinical Research Support Offices, which belong to the common departments of each hospital. In January 2017, these divisions and section were officially separated from the CRAS in the NCC organization.
In 2017, the Deputy Director was newly added to the CRAS organization on July 12. The Deputy Director supervises the whole CRAS by helping the Director and also holds the post of Chief of the Research Administration Division. The CRAS could have the Deputy Director, who has a pharmacology background and had careers of high positions in the Ministry of Health, Labour and Welfare (MHLW) and the Pharmaceuticals and Medical Devices Agency (PMDA). The Deputy Director has played a leading role to launch the Practical Research for Innovative Cancer Control Management Office (PRIMO) which offers various supports for the Practical Research for Innovative Cancer Control (PRICC) project in the
Japan Cancer Research Project by the Japan Agency for Medical Research and Development (AMED). In addition to the support of the PS/PO (Program Supervisor / Office) in the PRICC project, the
PRIMO manages the progress of the PRICC project as a whole, and offers investigator mapping, data mining of cancer research portfolio in Japan and abroad, consultations on intellectual properties and ELSI (ethical, legal and social issues). Although the PRIMO works for the PRICC investigators all over Japan, its functions overlap largely with those of the CRAS, and the CRAS staff has been involved deeply in the PRIMO project.
Another major structural evolution of the CRAS in 2017 was the establishment of the Bioethics Division by expanding the former Bioethics Section. The Clinical Trials Act was promulgated on April 14, 2017 and will come into effect on April 1, 2018. By the end of the period of the transitional measures, March 31, 2019, a great deal of actions and works would be required to this division. Despite the serious scarcity of the human resources in the specialty, the NCC has been endowed with strong staff, who has been contributing to not just inside the NCC but also all over Japan with regard to the research ethics-related capabilities.
(Future prospects)
The NCC embarked for the new era under the leadership of newly appointed President and Directors of the NCC Research Institute (NCCRI) and both hospitals (NCCH, NCCHE) on April 1, 2016. The Vision and high-priority research targets have been redefined, and re-organization is in progress in various parts of the NCC. However, the core concept of the CRAS stays unchanged to contribute to the NCC mission by promoting organic unity of the NCC as a whole. It is particularly important to respect differences in roles and characteristics of each NCC campus, Tsukiji and Kashiwa, to make the most of their strong points to maximize the research output of the NCC. Collaborations with the Clinical Research Support Offices of both hospitals remain crucial, and the CRAS wishes to play a significant role in bridging the various sections together in the NCC.
Activities and Future prospects of each Division/Section
1. Research administration Division
1) Research Administration Section
The Research Administration Section is a central office in charge of various administrative work related to research funding including application and reporting. The major external funding sources of the NCC are competitive grants from the government and government-supported agencies, such as the MHLW, the Japan Science and Technology Agency (JST), and the AMED.
This section also serves as an administrative office for the NCC Research and Development Fund, which is provided directly from the government to the NCC for fulfillment of its mission as the national core institute of the cancer control. This section organized seminars regarding research funding and its rules to prevent financial and scientific misconducts.
(Future prospects)
The "Guidelines for Managing and Auditing Public Research Funds at Research Institutes" and the "Guidelines for Responding to Misconduct in Research" were updated by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in February 2014 and adopted by the MHLW in March 2014. This section serves as a compliance promotion office of the Guidelines and has established a new system for research fund administration, which is fully compatible with the new Guidelines.
Furthermore, this section will support researchers to acquire competitive research funding by collecting and providing information on public research funding.
2) Research Alliance Section and Intellectual Property Section
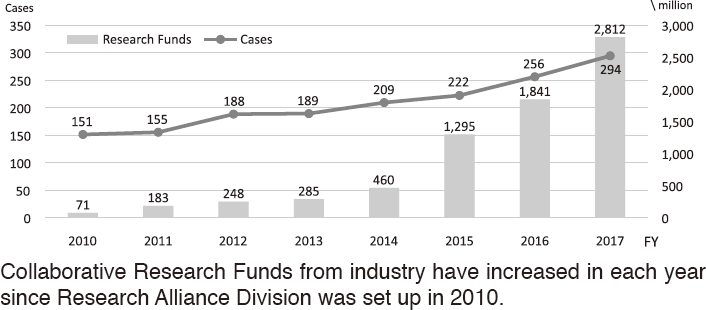
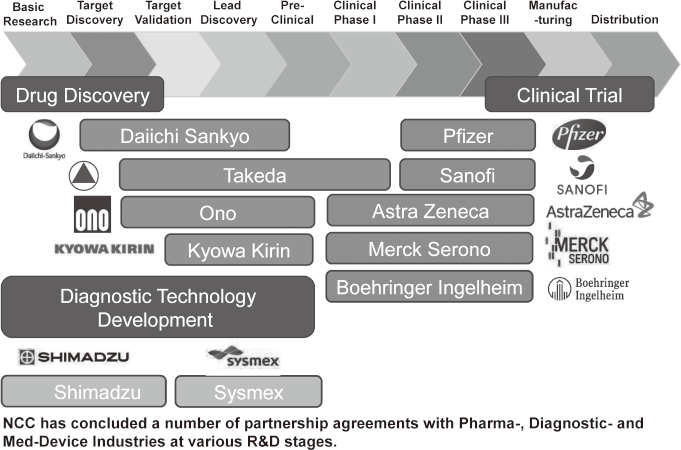
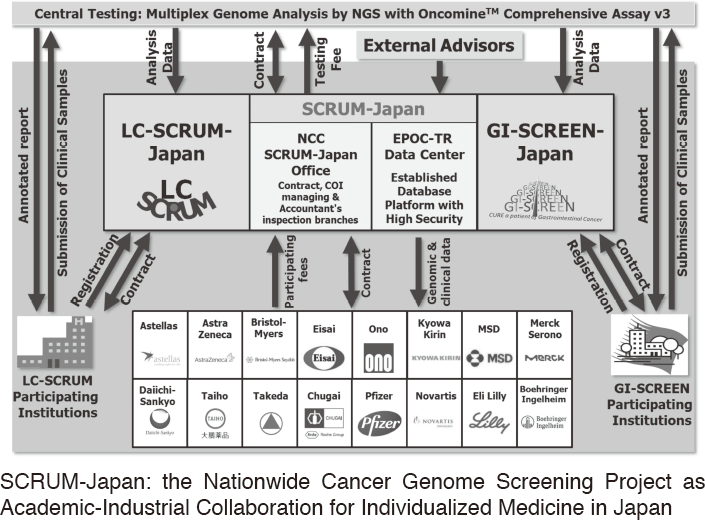
To make the NCC research outcomes clinically useful products available to cancer patients, the Research Alliance Section promotes collaborative research arrangements with private sectors. Number of collaborative research and their funds has been increasing in each year (Figure 1). In 2017, the number of collaboration was 294, and their research funds amount to ca. 2.6 billion yen, which exceeded those of 2016. The NCC has developed the comprehensive collaboration research system with companies and academic institutions. With the addition of a new collaborative framework this year, the NCC has 11 comprehensive collaborative alliances (Figure 2). This section supported a nationwide genomic screening project with the participation of major pharmaceutical companies and institutions across Japan (SCRUM-Japan "Cancer Genome Screening Project for Individualized Medicine in Japan"), which was launched in March 2015. Currently, 16 pharmaceutical companies are registered (Figure 3).
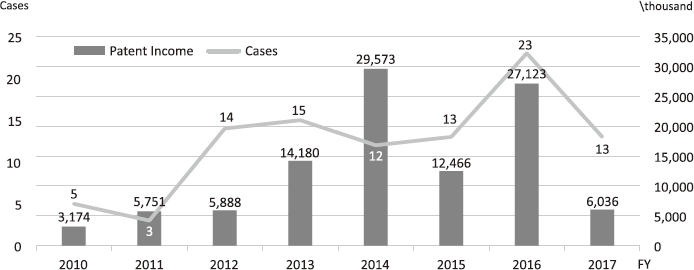
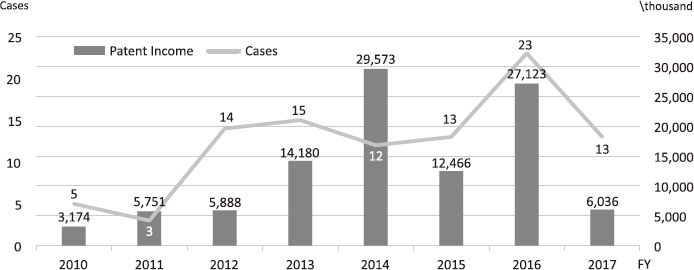
The Intellectual Property (IP) Section constantly reviews IPs and dismisses those that could not find a business sponsor within a certain period and the limited budget would be focused on IPs that are commercially viable. The number of the patent licensing in recent years is shown in Figure 4.
Staff members actively participate in seminars with regard to IP laws and regulations to update their knowledge and elevate their skills to promote academic-industrial alliances. Their competency in problem solution gained through on-the-job training (OJT) and effective consultation with experts will enable them to face new challenges in innovative affairs.
(Future prospects)
In the trend of the open innovation, this section will keep supporting creation of systemic and effective collaborative research frameworks. It also foresees the possibility of new laboratory setup where research is being performed by researchers from both industry and the NCC, and lead to more functional collaboration.
As to IP management, the NCC employs patent strategies to protect the potential value of the invention for industry, through which the translation of academic science and technology is made to the patient's bedside. The IP section plays an important role in assisting the NCC's comprehensive decision making, taking various aspects into consideration such as incubation of innovative technology, cost and effect balance, and risk management.
3) Research Administrators
Research administrators (RA) helped the director of the NCCRI to review the research achievements in 2017, and to develop 2018 and six-year research plans. RA also supported the director of the Strategic Planning Bureau to evaluate the mid-term progress of Cancer Research 10-year Strategy by government, and to propose research plans and operational improvements for the next-term of Strategy in collaboration with the AMED, MHLW, MEXT, and Ministry of Economy, Trade and Industry (METI).
RA has supported the promotion of the commercial viability of research outcomes based upon a comprehensive alliance with companies. Candidate compounds for clinical development have been provided by collaboration with business enterprises.
(Future prospects)
RA promotes an acquisition of large competitive research fund through a reinforcement of collaboration between organizations in the NCC, and also promotes translation of innovative research in the NCC to clinical diagnostic and therapeutic development and to patient care through four major mechanisms: the NCC Seeds Selection Committee, comprehensive alliances with leading companies, participation in the academic Drug Discovery Network, and establishment of NCC spin-off venture companies.
2. Biostatistics Division
The Biostatistics Division has a responsibile role in study design, analysis, interpretation, and publication, especially in the Japan Clinical Oncology Group (JCOG) and the Exploratory Oncology Research & Clinical Trial Center (EPOC) clinical trials and the investigator-initiated clinical trials which are led by investigators in the NCC Hospitals. We have also committed to establish an infrastructure to support clinical trials in the NCC Hospitals. Furthermore, we have been actively involved in collaborative relationships with the NCC Research Institute and the Center for Public Health Sciences.
We provided 14 introductory biostatistics lectures for investigators in the NCC to learn and review the elementary aspect of biostatistics. We newly provided the lecture about the implementation of statistical analysis with analysis software. We had a cumulative total of 495 participants. In addition, we hosted four biostatistics lectures to cover the important biostatistical side of various application fields and a cumulative total of 135 investigators participated. The lectures are open to any applicants from outside institutes.
Furthermore, we have provided biostatistical consultation and expertise, which supports NCC investigators working on basic, translational, clinical and epidemiological researches. We offered advice regarding 129 problems (77 in the Tsukiji Campus and 52 in the Kashiwa Campus) for which biostatistical consultation was requested during the period from January 2017 to March 2018.
(Future Prospects)
The NCC has a critical role for providing clinical service, education, conducting researches, and making policy recommendation/proposal, which all need decisions on the basis of solid and scientific evidence from reliable data and information. The mission of the Biostatistics Division is to contribute to providing the best evidence and the improvement of clinical practice and public health through the development and application of statistical methods. The Biostatistics Division is expanding on its independent and collaborative research within a range of areas, including prevention and policy recommendation/proposal, as well as treatment development. This year, we made particular effort to build effective relationships with investigators at the NCC Research Institute and the Center for Public Health Sciences. Collaborative projects have been launched, including the evaluation and implementation of medical technology with artificial intelligence (AI). Collaboration on epidemiologic studies has motivated to develop statistical methods to get deeper insight of etiological mechanisms or public health impact. We are working on promoting a cooperative framework with outside experts in statistics/biostatistics. We are opening up a new methodological research area in which a mathematical approach will serve as a solid basis.
3. Bioethics Division
1) Bioethics and Healthcare Law Section
In the Bioethics and Healthcare Law Section, we provide research ethics consultation services for researchers, research supporters, and Institutional Review Board (IRB) members for various situations from research design to public announcements. Research ethics consultations differ from an ethics review in that they are considered to be advice which holds no binding force, and which handles ethical issues not limited to regulatory compliance. In FY2017, we held 102 consultations. We also provide ethics education for researchers, IRB members, and IRB office staff.
(Future prospects)
We intend to continue providing research ethics consultation services. In FY2018 in particular, we anticipate a significant rise in consultations due to enforcement of the Clinical Trials Act. In addition to updates reflecting changes in the research environment, we plan to offer new ethics education content moving forward.
2) Human Research Protection Section
The Human Research Protection Section oversees affairs related to the obligations of chiefs of research facilities pertaining to ethics reviews, as well as administrative operations of review committees of various types for research involving human subjects. With regard to duties of committee administrative offices, the Human Research Protection Section handles the duties for the various types of IRBs.
Accomplishments of the IRB in FY2017 included 1,564 active research projects and 365 new research plans among the NCC faculty members' research, and a total of 110 new research plans from researchers outside of the NCC for which reviews were requested. Furthermore, the IRB was certified on March 31, 2017 by the Health Policy Bureau Director of the MHLW as the IRB with a high quality review system.
(Future prospects)
Since initiating research ethics consultation services, we have intended to offer rapid and sound reviews by treating the time spent on consultations with researchers as review work, and sharing consultation cases. We also plan to continue considering the state of appropriate and efficient review, in coordination with the NCCH Ethical Review Support Section and the NCCHE Ethical Review Support Section which oversee the review work of Certified Review Board as set forth in the Clinical Trials Act.
3) COI Management Section
As the administrative office of the Conflict of Interest (COI) committee, the COI Management Section responds to inquiries concerning COI and handles the administrative work pertaining to COI reviews for research faculty. The targets of COI review are research projects using public research funding, and a portion of clinical research. We also perform COI management for various committee members. In FY2017, we improved the efficiency of operations and revised the review workflow, smoothly executing COI reviews of 312 research projects (with researchers numbering 3,300 in total). We responded to eight requests for consultation from other departments. We also provide information and education for researchers concerning COI.
(Future prospects)
We aim for efficient operation of the COI review and management system while maintaining its quality and continually improving. As a change in flow of COI management is anticipated due to enforcement of the Clinical Trials Act in FY2018, we will strive to establish an efficient workflow and provide the latest information for researchers. We also plan to assess the consistency between COI management standards for research complying with ethical guidelines and COI management standards under the Clinical Trials Act.
Figure 1. Collaborative Research Trends in the NCC
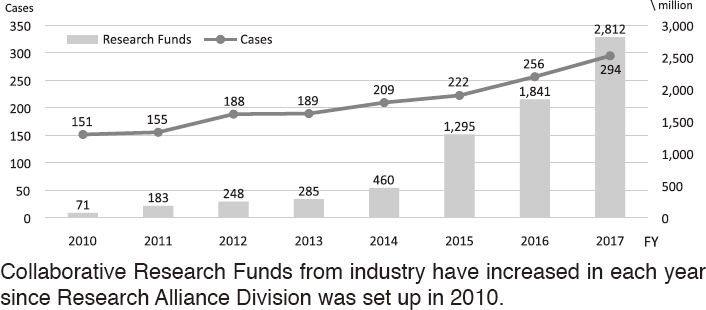
Figure 2. Major Industry Partners of the NCC
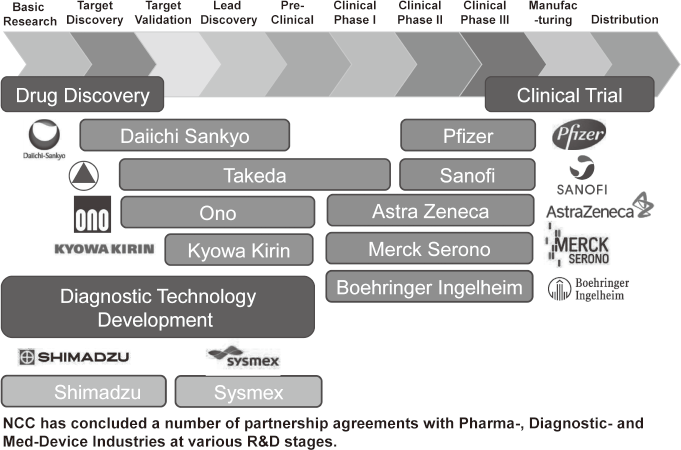
Figure 3. SCRUM-Japan
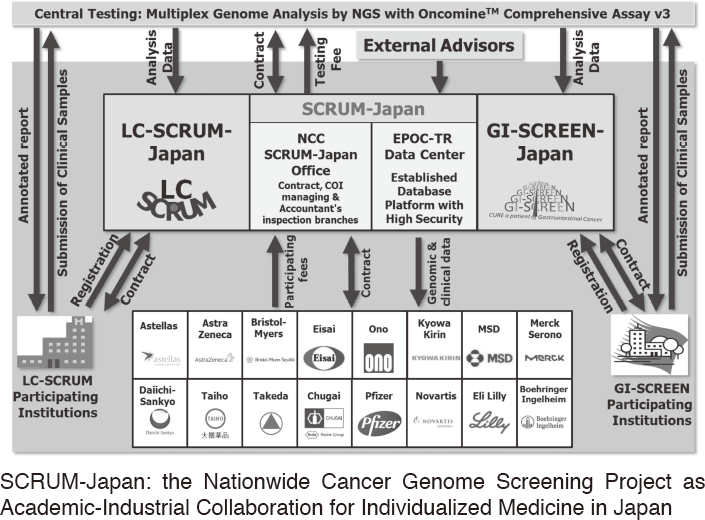
Figure 4. Licensing Trend in the NCC
List of papers published in January 2017 - March 2018
Journal
1. Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, Nashimoto A, Ito S, Kaji M, Imamura H, Fukushima N, Fujitani K. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg, 265:277-283, 2017
2. Ito S, Sano T, Mizusawa J, Takahari D, Katayama H, Katai H, Kawashima Y, Kinoshita T, Terashima M, Nashimoto A, Nakamori M, Onaya H, Sasako M. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric cancer, 20:322-331, 2017
3. Sekine I, Harada H, Yamamoto N, Wakabayashi M, Murakami H, Goto K, Nogami N, Seto T, Oshita F, Okamoto H, Tanaka H, Tamura T, Ishikura S, Ohe Y. Randomized phase II trial of weekly dose-intensive chemotherapy or amrubicin plus cisplatin chemotherapy following induction chemoradiotherapy for limited-disease small cell lung cancer (JCOG1011). Lung Cancer, 108:232-237, 2017
4. Aokage K, Saji H, Suzuki K, Mizutani T, Katayama H, Shibata T, Watanabe S, Asamura H. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg, 65:267-272, 2017
5. Aokage K, Miyoshi T, Ishii G, Kusumoto M, Nomura S, Katsumata S, Sekihara K, Hishida T, Tsuboi M. Clinical and Pathological Staging Validation in the Eighth Edition of the TNM Classification for Lung Cancer: Correlation between Solid Size on Thin-Section Computed Tomography and Invasive Size in Pathological Findings in the New T Classification. J Thorac Oncol, 12:1403-1412, 2017
6. Kurokawa Y, Yamaguchi T, Sasako M, Sano T, Mizusawa J, Nakamura K, Fukuda H. Institutional variation in short- and long-term outcomes after surgery for gastric or esophagogastric junction adenocarcinoma: correlative study of two randomized phase III trials (JCOG9501 and JCOG9502). Gastric cancer, 20:508-516, 2017
7. Ohue M, Iwasa S, Kanemitsu Y, Hamaguchi T, Shiozawa M, Ito M, Yasui M, Katayama H, Mizusawa J, Shimada Y. A Phase II/III randomized controlled trial comparing perioperative versus postoperative chemotherapy with mFOLFOX6 for lower rectal cancer with suspected lateral pelvic node metastasis: Japan Clinical Oncology Group Study JCOG1310 (PRECIOUS study). Jpn J Clin Oncol, 47:84-87, 2017
8. Ishiguro H, Saji S, Nomura S, Tanaka S, Ueno T, Onoue M, Iwata H, Yamanaka T, Sasaki Y, Toi M. A phase I/II pharmacokinetics/pharmacodynamics study of irinotecan combined with S-1 for recurrent/metastatic breast cancer in patients with selected UGT1A1 genotypes (the JBCRG-M01 study). Cancer Med, 6:2909-2917, 2017
9. Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, Yamaguchi T, Bandou H, Katsumata K, Murata K, Akagi Y, Takiguchi N, Saida Y, Nakamura K, Fukuda H, Akasu T, Moriya Y. Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg, 266:201-207, 2017
10. Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, Nunobe S, Fukushima N, Yasuda T, Asao Y, Fujiwara Y, Sasako M. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric cancer, 20:699-708, 2017
11. Namikawa K, Tsutsumida A, Mizutani T, Shibata T, Takenouchi T, Yoshikawa S, Kiyohara Y, Uchi H, Furue M, Ogata D, Tsuchida T, Yamazaki N. Randomized phase III trial of adjuvant therapy with locoregional interferon beta versus surgery alone in stage II/III cutaneous melanoma: Japan Clinical Oncology Group Study (JCOG1309, J-FERON). Jpn J Clin Oncol, 47:664-667, 2017
12. Nomura M, Kato K, Ando N, Ohtsu A, Muro K, Igaki H, Abe T, Takeuchi H, Daiko H, Gotoh M, Kataoka K, Wakabayashi M, Kitagawa Y. Comparison between neoadjuvant chemotherapy followed by surgery and definitive chemoradiotherapy for overall survival in patients with clinical Stage II/III esophageal squamous cell carcinoma (JCOG1406-A). Jpn J Clin Oncol, 47:480-486, 2017
13. Okuno T, Wakabayashi M, Kato K, Shinoda M, Katayama H, Igaki H, Tsubosa Y, Kojima T, Okabe H, Kimura Y, Kawano T, Kosugi S, Toh Y, Kato H, Nakamura K, Fukuda H, Ishikura S, Ando N, Kitagawa Y. Esophageal stenosis and the Glasgow Prognostic Score as independent factors of poor prognosis for patients with locally advanced unresectable esophageal cancer treated with chemoradiotherapy (exploratory analysis of JCOG0303). Int J Clin Oncol, 22:1042-1049, 2017
14. Iwasaki M, Tanaka-Mizuno S, Kuchiba A, Yamaji T, Sawada N, Goto A, Shimazu T, Sasazuki S, Wang H, Marchand LL, Tsugane S. Inclusion of a Genetic Risk Score into a Validated Risk Prediction Model for Colorectal Cancer in Japanese Men Improves Performance. Cancer Prev Res (Phila), 10:535-541, 2017
15. Kataoka K, Nakamura K, Mizusawa J, Kato K, Eba J, Katayama H, Shibata T, Fukuda H. Surrogacy of progression-free survival (PFS) for overall survival (OS) in esophageal cancer trials with preoperative therapy: Literature-based meta-analysis. Eur J Surg Oncol, 43:1956-1961, 2017
16. Hatogai K, Fujii S, Kojima T, Daiko H, Nomura S, Doi T, Kitano S, Ohtsu A, Takiguchi Y, Yoshino T, Ochiai A. Large-scale comprehensive immunohistochemical biomarker analyses in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol, 143:2351-2361, 2017
17. Kataoka K, Takeuchi H, Mizusawa J, Igaki H, Ozawa S, Abe T, Nakamura K, Kato K, Ando N, Kitagawa Y. Prognostic Impact of Postoperative Morbidity After Esophagectomy for Esophageal Cancer: Exploratory Analysis of JCOG9907. Ann Surg, 265:1152-1157, 2017
18. Kitano S, Inomata M, Mizusawa J, Katayama H, Watanabe M, Yamamoto S, Ito M, Saito S, Fujii S, Konishi F, Saida Y, Hasegawa H, Akagi T, Sugihara K, Yamaguchi T, Masaki T, Fukunaga Y, Murata K, Okajima M, Moriya Y, Shimada Y. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomised controlled trial. The lancet. Gastroenterology & hepatology, 2:261-268, 2017
19. Onimaru R, Onishi H, Shibata T, Hiraoka M, Ishikura S, Karasawa K, Matsuo Y, Kokubo M, Shioyama Y, Matsushita H, Ito Y, Shirato H. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer (JCOG0702): Results for the group with PTV100cc. Radiother Oncol, 122:281-285, 2017
20. Kimura T, Nagata Y, Eba J, Ozawa S, Ishikura S, Shibata T, Ito Y, Hiraoka M, Nishimura Y. A randomized Phase III trial of comparing two dose-fractionations stereotactic body radiotherapy (SBRT) for medically inoperable Stage IA non-small cell lung cancer or small lung lesions clinically diagnosed as primary lung cancer: Japan Clinical Oncology Group Study JCOG1408 (J-SBRT trial). Jpn J Clin Oncol, 47:277-281, 2017
21. Tachibana S, Miyamoto S, Goto T, Ishida K, Iida T, Okazaki M, Yoshida S, Nomura S, Hayashi R, Sakuraba M. Efficacy of Tensed and Straight Free Jejunum Transfer for the Reduction of Postoperative Dysphagia. Plastic and reconstructive surgery. Global open, 5:e1599, 2017
22. Shitara K, Doi T, Nagano O, Fukutani M, Hasegawa H, Nomura S, Sato A, Kuwata T, Asai K, Einaga Y, Tsuchihashi K, Suina K, Maeda Y, Saya H, Ohtsu A. Phase 1 study of sulfasalazine and cisplatin for patients with CD44v-positive gastric cancer refractory to cisplatin (EPOC1407). Gastric cancer, 20:1004-1009, 2017
23. Nomura S, Hirakawa A, Hamada C. Sample size determination for the current strategy in oncology Phase 3 trials that tests progression-free survival and overall survival in a two-stage design framework. J Biopharm Stat, 1-23, 2017
24. Shitara K, Doi T, Nagano O, Imamura CK, Ozeki T, Ishii Y, Tsuchihashi K, Takahashi S, Nakajima TE, Hironaka S, Fukutani M, Hasegawa H, Nomura S, Sato A, Einaga Y, Kuwata T, Saya H, Ohtsu A. Dose-escalation study for the targeting of CD44v+ cancer stem cells by sulfasalazine in patients with advanced gastric cancer (EPOC1205). Gastric Cancer, 20:341-349, 2017
25. Yoh K, Seto T, Satouchi M, Nishio M, Yamamoto N, Murakami H, Nogami N, Matsumoto S, Kohno T, Tsuta K, Tsuchihara K, Ishii G, Nomura S, Sato A, Ohtsu A, Ohe Y, Goto K. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med, 5:42-50, 2017
26. Yano T, Yoda Y, Nomura S, Toyosaki K, Hasegawa H, Ono H, Tanaka M, Morimoto H, Horimatsu T, Nonaka S, Kaneko K, Sato A. Prospective trial of biodegradable stents for refractory benign esophageal strictures after curative treatment of esophageal cancer. Gastrointest Endosc, 86:492-499, 2017
27. Drew DA, Nishihara R, Lochhead P, Kuchiba A, Qian ZR, Mima K, Nosho K, Wu K, Wang M, Giovannucci E, Fuchs CS, Chan AT, Ogino S. A Prospective Study of Smoking and Risk of Synchronous Colorectal Cancers. Am J Gastroenterol, 112:493-501, 2017
28. Yamada M, Oda I, Tanaka H, Abe S, Nonaka S, Suzuki H, Yoshinaga S, Kuchiba A, Koyanagi K, Igaki H, Taniguchi H, Sekine S, Saito Y, Tachimori Y. Tumor location is a risk factor for lymph node metastasis in superficial Barrett's adenocarcinoma. Endoscopy international open, 5:E868-E874, 2017
29. Inokuchi J, Kuroiwa K, Kakehi Y, Sugimoto M, Tanigawa T, Fujimoto H, Gotoh M, Masumori N, Ogawa O, Eto M, Ohyama C, Yamaguchi A, Matsuyama H, Ichikawa T, Asano T, Mizusawa J, Eba J, Naito S. Role of lymph node dissection during radical nephroureterectomy for upper urinary tract urothelial cancer: multi-institutional large retrospective study JCOG1110A. World J Urol, 35:1737-1744, 2017
30. Shinozaki E, Yoshino T, Yamazaki K, Muro K, Yamaguchi K, Nishina T, Yuki S, Shitara K, Bando H, Mimaki S, Nakai C, Matsushima K, Suzuki Y, Akagi K, Yamanaka T, Nomura S, Fujii S, Esumi H, Sugiyama M, Nishida N, Mizokami M, Koh Y, Abe Y, Ohtsu A, Tsuchihara K. Clinical significance of BRAF non-V600E mutations on the therapeutic effects of anti-EGFR monoclonal antibody treatment in patients with pretreated metastatic colorectal cancer: the Biomarker Research for anti-EGFR monoclonal Antibodies by Comprehensive Cancer genomics (BREAC) study. Br J Cancer, 117:1450-1458, 2017
31. Enokida T, Fujii S, Takahashi M, Higuchi Y, Nomura S, Wakasugi T, Yamazaki T, Hayashi R, Ohtsu A, Tahara M. Gene expression profiling to predict recurrence of advanced squamous cell carcinoma of the tongue: discovery and external validation. Oncotarget, 8:61786-61799, 2017
32. Watari H, Katayama H, Shibata T, Ushijima K, Satoh T, Onda T, Aoki D, Fukuda H, Yaegashi N, Sakuragi N. Phase III trial to confirm the superiority of pelvic and para-aortic lymphadenectomy to pelvic lymphadenectomy alone for endometrial cancer: Japan Clinical Oncology Group Study 1412 (SEPAL-P3). Jpn J Clin Oncol, 47:986-990, 2017
33. Takahari D, Mizusawa J, Koizumi W, Hyodo I, Boku N. Validation of the JCOG prognostic index in advanced gastric cancer using individual patient data from the SPIRITS and G-SOX trials. Gastric cancer, 20:757-763, 2017
34. Goto M, Naito M, Saruwatari K, Hisakane K, Kojima M, Fujii S, Kuwata T, Ochiai A, Nomura S, Aokage K, Hishida T, Yoshida J, Yokoi K, Tsuboi M, Ishii G. The ratio of cancer cells to stroma after induction therapy in the treatment of non-small cell lung cancer. J Cancer Res Clin Oncol, 143:215-223, 2017
35. Aokage K, Okada M, Suzuki K, Nomura S, Suzuki S, Tsubokawa N, Mimae T, Hattori A, Hishida T, Yoshida J, Tsuboi M. Is cancer history really an exclusion criterion for clinical trial of lung cancer? Influence of gastrointestinal tract cancer history on the outcomes of lung cancer surgery. Jpn J Clin Oncol, 47:145-156, 190, 2017
36. Aokage K, Miyoshi T, Ishii G, Kusumoto M, Nomura S, Katsumata S, Sekihara K, Tane K, Tsuboi M. Influence of Ground Glass Opacity and the Corresponding Pathological Findings on Survival in Patients with Clinical Stage I Non-Small Cell Lung Cancer. J Thorac Oncol, 13:533-542, 2018
37. Budhathoki S, Hidaka A, Yamaji T, Sawada N, Tanaka-Mizuno S, Kuchiba A, Charvat H, Goto A, Kojima S, Sudo N, Shimazu T, Sasazuki S, Inoue M, Tsugane S, Iwasaki M. Plasma 25-hydroxyvitamin D concentration and subsequent risk of total and site specific cancers in Japanese population: large case-cohort study within Japan Public Health Center-based Prospective Study cohort. BMJ, 360:k671, 2018
38. Kodaira T, Kagami Y, Shibata T, Shikama N, Nishimura Y, Ishikura S, Nakamura K, Saito Y, Matsumoto Y, Teshima T, Ito Y, Akimoto T, Nakata K, Toshiyasu T, Nakagawa K, Nagata Y, Nishimura T, Uno T, Kataoka M, Yorozu A, Hiraoka M. Results of a multi-institutional, randomized, non-inferiority, phase III trial of accelerated fractionation versus standard fractionation in radiation therapy for T1-2N0M0 glottic cancer: Japan Clinical Oncology Group Study (JCOG0701). Ann Oncol, 29:992-997, 2018
39. Taguri M, Kuchiba A. Decomposition of the population attributable fraction for two exposures. Ann Epidemiol, 28:331-334, 2018
40. Hishida T, Saji H, Watanabe SI, Asamura H, Aokage K, Mizutani T, Wakabayashi M, Shibata T, Okada M. A randomized Phase III trial of lobe-specific vs. systematic nodal dissection for clinical Stage I-II non-small cell lung cancer (JCOG1413). Jpn J Clin Oncol, 48:190-194, 2018
Book
1. Kuchiba A. Evaluation of Cancer Risk in Epidemiologic Studies with Genetic and Molecular Data. In: Matsui S, Crowley J (ed), Frontiers of Biostatistical Methods and Applications in Clinical Oncology, 2017