Annual Report 2017
Division of Carcinogenesis and Cancer Prevention(Viral Carcinogenesis and Cancer Prevention Group)
Tohru Kiyono, Takashi Yugawa, Tomomi Nakahara, Ghani Farhana Ishrat, Takako Ishiyama, , Katsuyuki Tanaka, Chiho Kohno, Yukimi Gotoh, Etsuko Kabasawa, Kenji Yamada, Yuki Inagawa, Shin-ichi Ohno
Introduction
Approximately 15% of human cancers have a viral etiology. Aims of research in Viral Carcinogenesis and Cancer Prevention Group is to elucidate the molecular mechanisms for carcinogenesis and viral persistence of human papillomavirus (HPV) infection in order to develop effective cancer prevention. Persistent infection of oncogenic HPVs is responsible for 5% of all cancers worldwide including cervical, anal, vaginal, vulvar, penile, and head and neck cancers. The E6 and E7 proteins of oncogenic HPVs are known to inactivate the major tumor suppressors, p53 and retinoblastoma protein (pRB), respectively. By using an in vitro multistep carcinogenesis model, we study specific association between oncogenic driver genes and pathological features of cancers with and without a viral etiology.
Our team and what we do
To study molecular pathology of cancers with or without viral etiology, we are developing ex vivo carcinogenesis models by transducing genetic abnormalities frequently found in given cancers into their respective normal cells-of-origin. With our tissue culture models, we aim to (1) elucidate mechanisms of viral persistence of HPV infection and (2) analyze association between driver mutations and cancer characteristics.
Research activities
1. HPV-induced carcinogenesis and its prevention
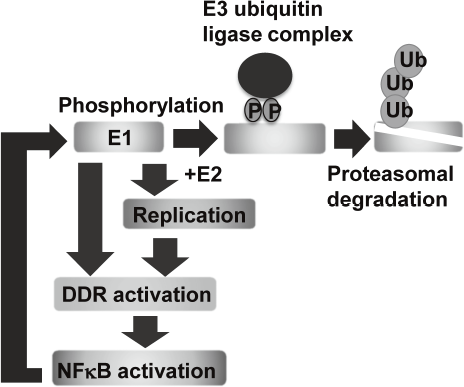
Eradication of viral genomes and/or the infected cells from persistently infected tissues can realize non-invasive treatment to prevent HPV associated cancers. To develop new strategies for viral elimination, it is critical to gain insight into the molecular mechanisms of the viral genome replication. HPV life cycle depends on the differentiation of the stratified epithelium. In basal cells, viral genomes are maintained as low copy number-episomes and expression of the viral genes is minimal. This latent state is thought to facilitate persistent infection by eluding host immune surveillance. We established a tissue culture model which can recapitulate HPV life cycle and demonstrated that the viral helicase, E1 is dispensable for maintaining HPV episomes in basal cells. Subsequent studies indicated that E1 expression induces activation of NFkB pathway through DNA damage response (DDR) and NFκB functions as a negative feedback by facilitating phosphorylation-dependent proteasomal degradation of E1. We propose that this negative feedback loop is a regulatory mechanism to limit an E1 level in basal cells and necessary for sustaining the latent state of HPV infection (Figure 1).
Figure 1. E1-NFkB negative feedback loop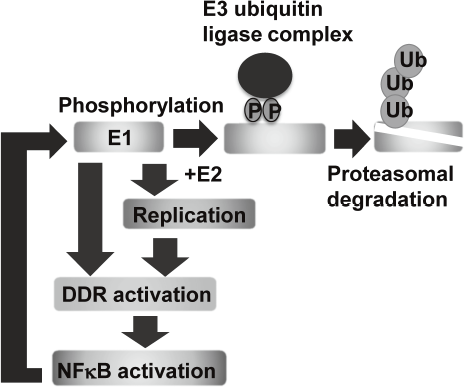
We further investigated a possible link between disruption of the E1-NFκB feedback loop and neoplastic progression of cervical lesions.
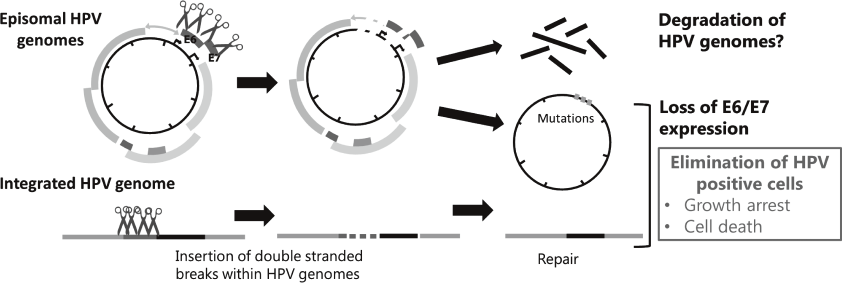
Genome editing technologies such as CRISPR/Cas9 made it possible to directly target HPV genome. We also began to test effectiveness of targeting HPV genomes by the CRISPR/Cas9 system to eliminate viral genomes and/or the infected cells (Figure 2).
Figure 2. Strategies for eradication of HPV genomes and/or HPV positive cells with CRISPR/Cas9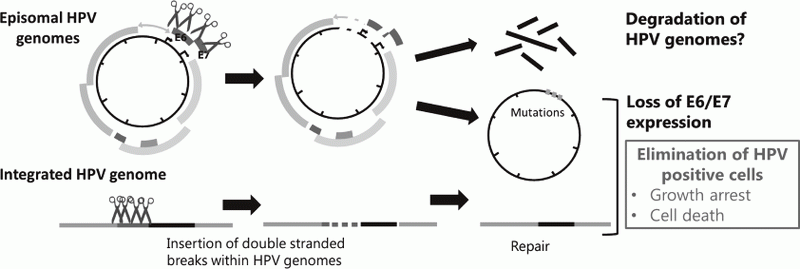
2. In vitro human carcinogenesis model
We have been studying the biological significance of ΔNp63α (hereafter just p63) expression and the molecular basis underlying acquisition of malignant traits in squamous cell carcinomas (SCCs) of cervical and head and neck cancers. p63 is a master regulator for the development of stratified epithelium and has a role in stem cell maintenance. In line with its physiological role, p63 is frequently overexpressed in more than 50% of human SCCs including examples in the cervix, lung, and head and neck. Paradoxically, loss of p63 is shown to be associated with metastasis appearance and poor prognosis in SCCs, although the underlying molecular mechanism and its functional relevance to carcinogenesis remains unclear as is the case with NOTCH1. We have identified the novel NOTCH-ROCK pathway downstream of p63. Using our in vitro human multistep carcinogenesis model for cervical cancer, we revealed that knock-down of p63 conferred increased invasiveness through the NOTCH-ROCK pathway in MYC-overexpressing cells (Figure 3). We also confirmed the tendency of mutual exclusivity between p63 and MYC expressions in head and neck SCCs by TCGA analysis. Current studies involve identification of downstream effectors in the NOTCH-ROCK pathway and its activation mechanisms. Our goal is to develop the novel strategies for diagnosis and molecularly targeted therapy against poorly-differentiated SCCs based on its cancer biology.
Education
A PhD student from Thailand, a medical doctor from China who applied for the PhD program in a Japanese graduate school and two Japanese undergraduate students studied as trainees in our laboratory. A Japanese student had completed the PhD program and obtained a PhD from the affiliated graduate school (Tokyo Medical and Dental University).
Future prospects
On the basis of our findings that uncover a molecular mechanism for viral persistence, we hope to develop a new approach for eradication of HPV genome and/or HPV-infected cells. The in vitro carcinogenesis model for any given cancer can be established in vitro with our techniques. These models will be useful for preclinical assessment of new cancer therapies.
Figure 3. Proposed model for the NOTCH-ROCK pathway and its biological significance in squamous cell carcinomas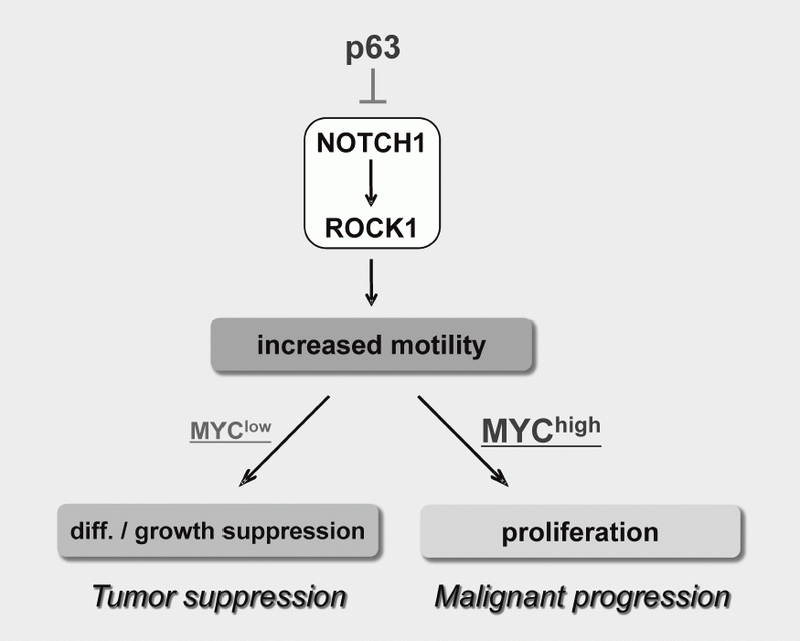
List of papers published in January 2017 - March 2018
Journal
1. Noguchi K, Wakai K, Kiyono T, Kawabe M, Yoshikawa K, Hashimoto-Tamaoki T, Kishimoto H, Nakano Y. Molecular analysis of keratocystic odontogenic tumor cell lines derived from sporadic and basal cell nevus syndrome patients. Int J Oncol, 51:1731-1738, 2017
2. Hashimoto N, Kiyono T, Saitow F, Asada M, Yoshida M. Reversible differentiation of immortalized human bladder smooth muscle cells accompanied by actin bundle reorganization. PLoS One, 12:e0186584, 2017
3. Yokoi A, Yoshioka Y, Yamamoto Y, Ishikawa M, Ikeda SI, Kato T, Kiyono T, Takeshita F, Kajiyama H, Kikkawa F, Ochiya T. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat Commun, 8:14470, 2017
4. Katayama M, Hirayama T, Kiyono T, Onuma M, Tani T, Takeda S, Nishimori K, Fukuda T. Immortalized prairie vole-derived fibroblasts (VMF-K4DTs) can be transformed into pluripotent stem cells and provide a useful tool with which to determine optimal reprogramming conditions. J Reprod Dev, 63:311-318, 2017
5. Shinohara M, Sumino Y, Sato F, Kiyono T, Hashimoto N, Mimata H. Tumor necrosis factor-alpha inhibits differentiation of myogenic cells in human urethral rhabdosphincter. Int J Urol, 24:461-467, 2017
6. Yamamoto E, Niimi K, Kiyono T, Yamamoto T, Nishino K, Nakamura K, Kotani T, Kajiyama H, Shibata K, Kikkawa F. Establishment and characterization of cell lines derived from complete hydatidiform mole. Int J Mol Med, 40:614-622, 2017
7. Nagata Y, Kiyono T, Okamura K, Goto YI, Matsuo M, Ikemoto-Uezumi M, Hashimoto N. Interleukin-1beta (IL-1beta)-induced Notch ligand Jagged1 suppresses mitogenic action of IL-1beta on human dystrophic myogenic cells. PLoS One, 12:e0188821, 2017
8. Hashimoto H, Suda Y, Miyashita T, Ochiai A, Tsuboi M, Masutomi K, Kiyono T, Ishii G. A novel method to generate single-cell-derived cancer-associated fibroblast clones. J Cancer Res Clin Oncol, 143:1409-1419, 2017
9. Kayama K, Watanabe S, Takafuji T, Tsuji T, Hironaka K, Matsumoto M, Nakayama KI, Enari M, Kohno T, Shiraishi K, Kiyono T, Yoshida K, Sugimoto N, Fujita M. GRWD1 negatively regulates p53 via the RPL11-MDM2 pathway and promotes tumorigenesis. EMBO Rep, 18:123-137, 2017
10. Fuchigami T, Koyama H, Kishida M, Nishizawa Y, Iijima M, Kibe T, Ueda M, Kiyono T, Maniwa Y, Nakamura N, Kishida S. Fibroblasts promote the collective invasion of ameloblastoma tumor cells in a 3D coculture model. FEBS open bio, 7:2000-2007, 2017
11. Yoshimatsu Y, Nakahara T, Tanaka K, Inagawa Y, Narisawa-Saito M, Yugawa T, Ohno SI, Fujita M, Nakagama H, Kiyono T. Roles of the PDZ-binding motif of HPV 16 E6 protein in oncogenic transformation of human cervical keratinocytes. Cancer Sci, 108:1303-1309, 2017
12. Mori S, Takeuchi T, Ishii Y, Yugawa T, Kiyono T, Nishina H, Kukimoto I. Human Papillomavirus 16 E6 Upregulates APOBEC3B via the TEAD Transcription Factor. J Virol, 91:2017
13. Kasahara K, Aoki H, Kiyono T, Wang S, Kagiwada H, Yuge M, Tanaka T, Nishimura Y, Mizoguchi A, Goshima N, Inagaki M. EGF receptor kinase suppresses ciliogenesis through activation of USP8 deubiquitinase. Nat Commun, 9:758, 2018
14. Nakamura K, Nakayama K, Ishikawa N, Ishikawa M, Sultana R, Kiyono T, Kyo S. Reconstitution of high-grade serous ovarian carcinoma from primary fallopian tube secretory epithelial cells. Oncotarget, 9:12609-12619, 2018
15. Dendo K, Yugawa T, Nakahara T, Ohno SI, Goshima N, Arakawa H, Kiyono T. Induction of non-apoptotic programmed cell death by oncogenic RAS in human epithelial cells and its suppression by MYC overexpression. Carcinogenesis, 39:202-213, 2018
