Annual Report 2017
Division of Cancer Stem Cell
Kenkichi Masutomi, Yoshinari Ando, Mami Yasukawa, Marco Ghilotti, Michie Yoshikawa, Tomoko Hayakawa, Yoshiko Maida
Introduction
Research in the Division of Cancer Stem Cell is focused on deciphering the mechanisms that establish and maintain cancer stem cells and developing novel therapeutic approaches to treat cancer stem cells. In particular, our division studies the molecular links among a) telomerase and RNA-dependent RNA polymerase (RdRP); b) TERT and cancer stem cells; and c) RdRP and anticancer drugs.
Research activities
1. Telomerase and RNA-dependent RNA polymerase
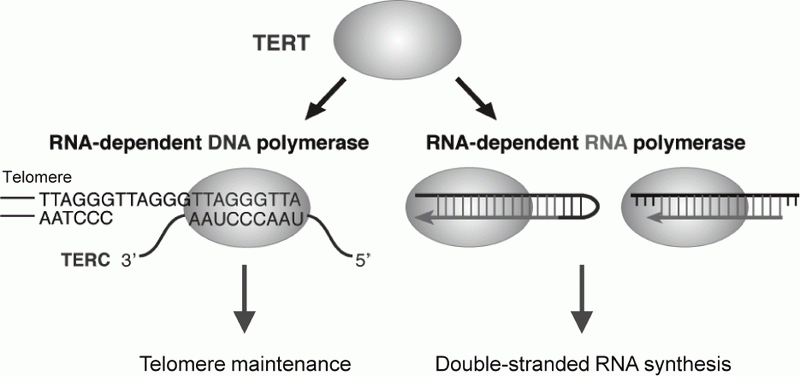
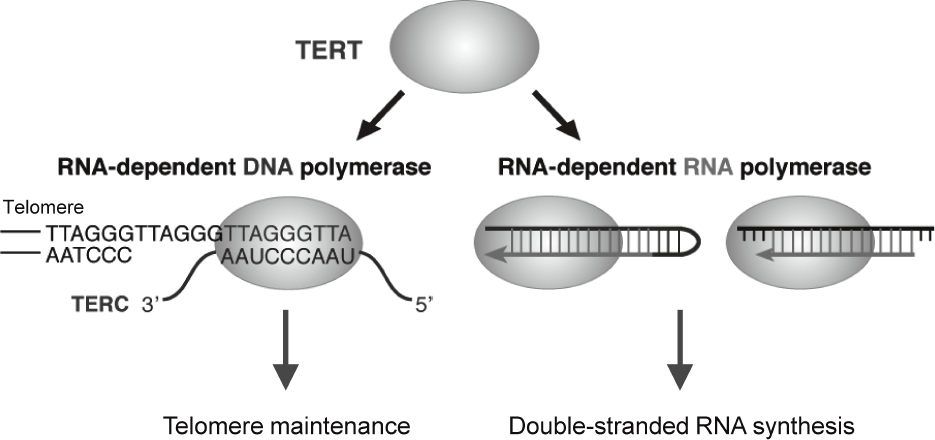
Telomerase is a ribonucleoprotein complex that elongates telomeres. Human telomerase reverse transcriptase (TERT) is known as the catalytic subunit of telomerase and acts as an RNA-dependent DNA polymerase (RdDP), which synthesizes telomere DNA repeats from an RNA template TERC. Although the major function of TERT is believed to be telomere elongation, emerging evidences indicate that TERT exhibits various functions beyond telomere maintenance. We reported that TERT has an RNA-dependent RNA polymerase (RdRP) activity and synthesizes double-stranded RNA (dsRNA) in either
a primer-dependent or a primer-independent
manner1, 2 (Figure 1). TERT assembles with BRG1 and nucleostemin (NS), and the TERT-BRG1-NS complex (TBN complex) exerts RdRP activity3. We found that the TBN complex contributes to mitotic progression through the regulation of
heterochromatin maintenance3. Our recent studies
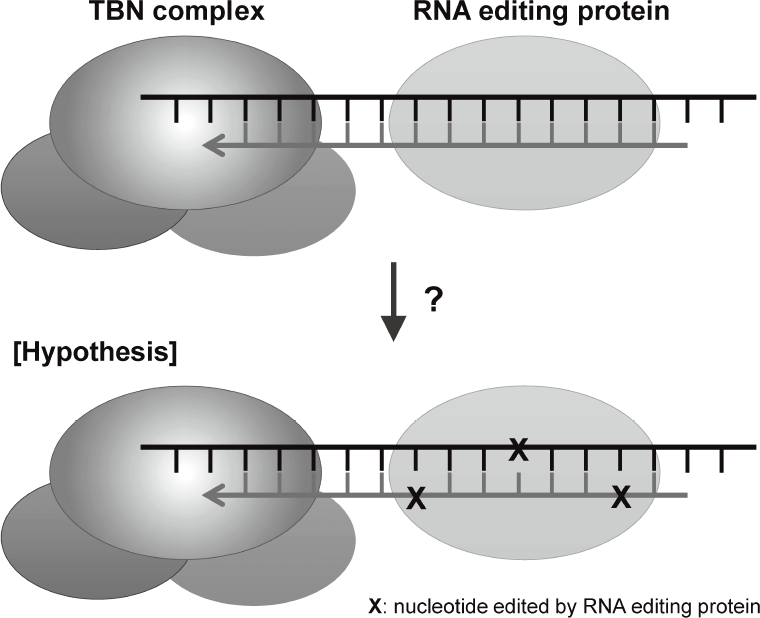
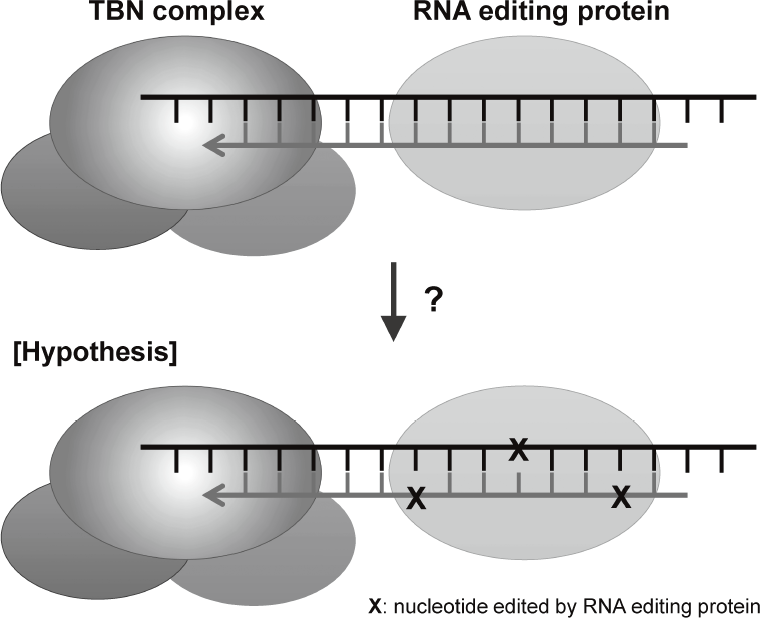
are disclosing that TERT-RdRP functionally interacts with RNA editing protein through dsRNAs (Figure 2).
2. TERT and cancer stem cells
Previous studies indicated that TERT has activities beyond telomere maintenance, and it is speculated that the constitutive expression of TERT not only stabilizes telomere length and facilitates cell immortalization, but also contributes to tumor susceptibility and alters stem cell cycling in vivo even when telomere lengths are not limited. We found that the TBN complex participates in the regulation of tumor initiating cells
(TICs) phenotypes through telomere-independent
mechanisms4.
3. RdRP and anticancer drugs
We found that eribulin mesylate (eribulin) effectively inhibits the growth of several cancer cells with elevated levels of TERT expression5. Although it has been confirmed that eribulin exerts its anticancer effect by blocking the elongation of microtubules, we found that eribulin specifically inhibits the RdRP activity of TERT in vitro, suggesting TERT-RdRP activity as a novel molecular target of eribulin. Our study demonstrated that eribulin might be a promising therapeutic agent for several cancers.
We further confirmed that the expression levels of TERT protein and the RdRP activities are positively correlated in various human cancer cell lines2, indicating that RdRP inhibitors may work effectively for many types of tumors with high levels of TERT expression. We have initiated a project to discover and characterize novel inhibitors of TERT-RdRP activity as anticancer drugs.
References
1. Maida Y et al. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature, 461:230-235, 2009.
2. Maida Y et al. De novo RNA synthesis by RNA-dependent RNA polymerase activity of TERT. Mol Cell Biol, 36:1248-1259, 2016
3. Maida Y et al. Involvement of telomerase reverse transcriptase in heterochromatin maintenance. Mol Cell Biol, 34:1576-1593, 2014.
4. Okamoto N et al. Maintenance of tumor initiating cells of defined genetic composition by nucleostemin. Proc Natl Acad Sci U S A, 108: 20388-20393, 2011.
5. Yamaguchi S et al. Eribulin mesylate targets human telomerase reverse transcriptase in ovarian cancer cells. PLOS ONE, 9:e112438, 2014.
List of papers published in January 2017 - March 2018
Journal
1. Hashimoto H, Suda Y, Miyashita T, Ochiai A, Tsuboi M, Masutomi K, Kiyono T, Ishii G. A novel method to generate single-cell-derived cancer-associated fibroblast clones. J Cancer Res Clin Oncol, 143:1409-1419, 2017
2. Ishibashi M, Neri S, Hashimoto H, Miyashita T, Yoshida T, Nakamura Y, Udagawa H, Kirita K, Matsumoto S, Umemura S, Yoh K, Niho S, Tsuboi M, Masutomi K, Goto K, Ochiai A, Ishii G. CD200-positive cancer associated fibroblasts augment the sensitivity of Epidermal Growth Factor Receptor mutation-positive lung adenocarcinomas to EGFR Tyrosine kinase inhibitors. Sci Rep, 7:46662, 2017
3. Masui K, Komori T, Kato Y, Masutomi K, Ichimura K, Ogasawara S, Kaneko MK, Oki H, Suzuki H, Nitta M, Maruyama T, Muragaki Y, Kawamata T, Sawada T, Shibata N. Elevated TERT Expression in TERT-Wildtype Adult Diffuse Gliomas: Histological Evaluation with a Novel TERT-Specific Antibody. Biomed Res Int, 2018:7945845, 2018
