Annual Report 2017
Division of Cancer Immunology
Hiroyoshi Nishikawa, Yuka Maeda, Hitomi Nishinakamura, Kentaro Shinohara, Hiroaki Hiramatsu, Etsuko Tanji, Yuki Ishige, Maki Sugaya, Akihito Maki, Yusuke Muto, Nanae Shimoyama
Introduction
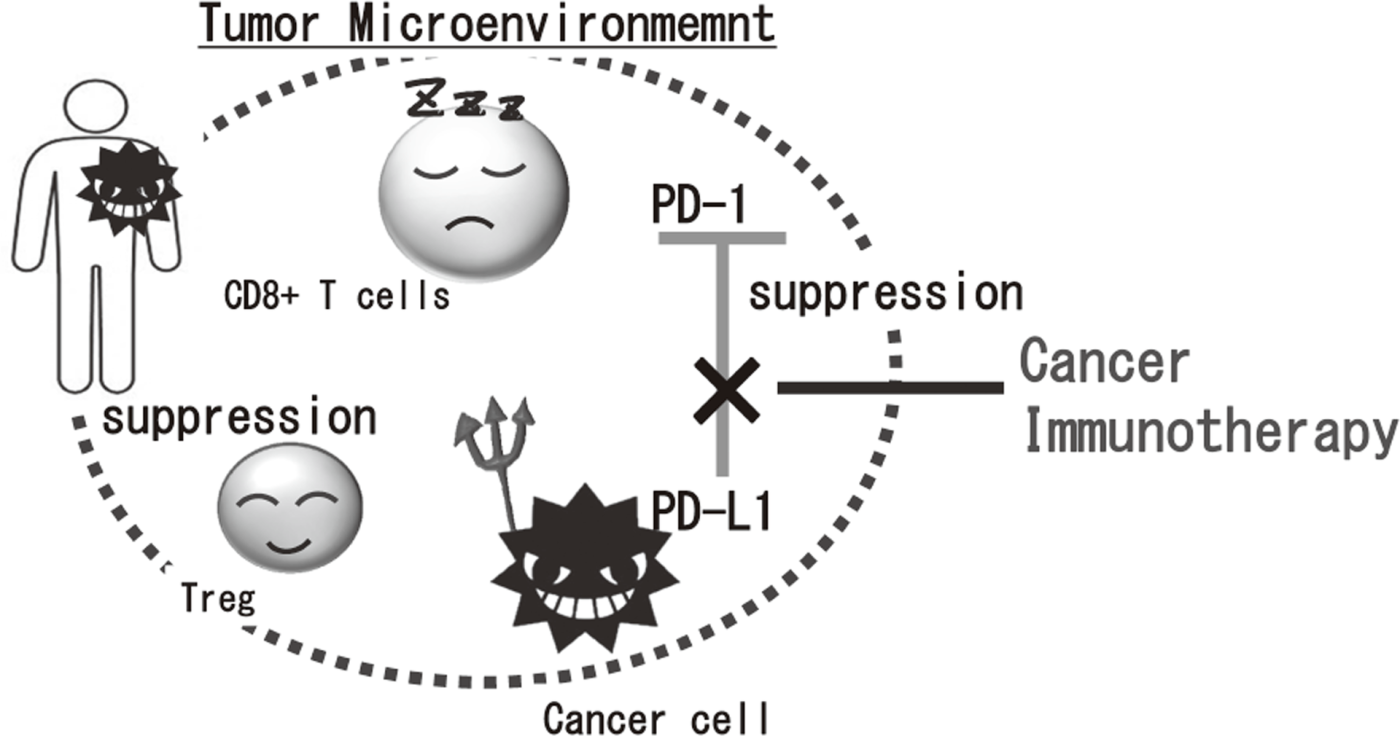
Cancer immunotherapies, particularly im- mune checkpoint inhibitors such as anti-CTLA-4 mAb, anti-PD-1 mAb, and anti-PD-L1 mAb, are becoming important strategies to treat cancer. Yet, the clinical effects of immune checkpoint inhibitor are observed in limited patients because cancer cells establish a complexed immune suppressive mechanism that inhibit effective anti-tumor immune responses. To reveal the mechanisms is an urgent requirement for the development of effective cancer immunotherapy (Figure 1). Our aims are clarifying the detailed mechanism of immune suppression in tumor microenvironment by immunologic and genetic approaches and identifying novel approaches to control anti-tumor immune responses.
Figure 1. Suppression of anti-tumor immune responses in tumor microenvironment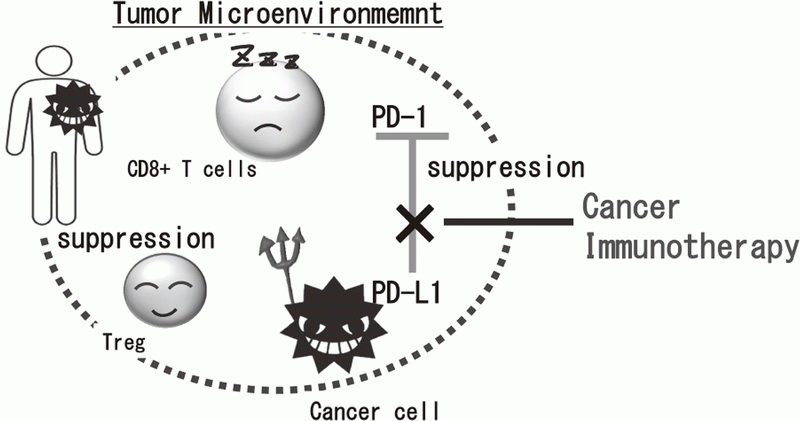
Research activities
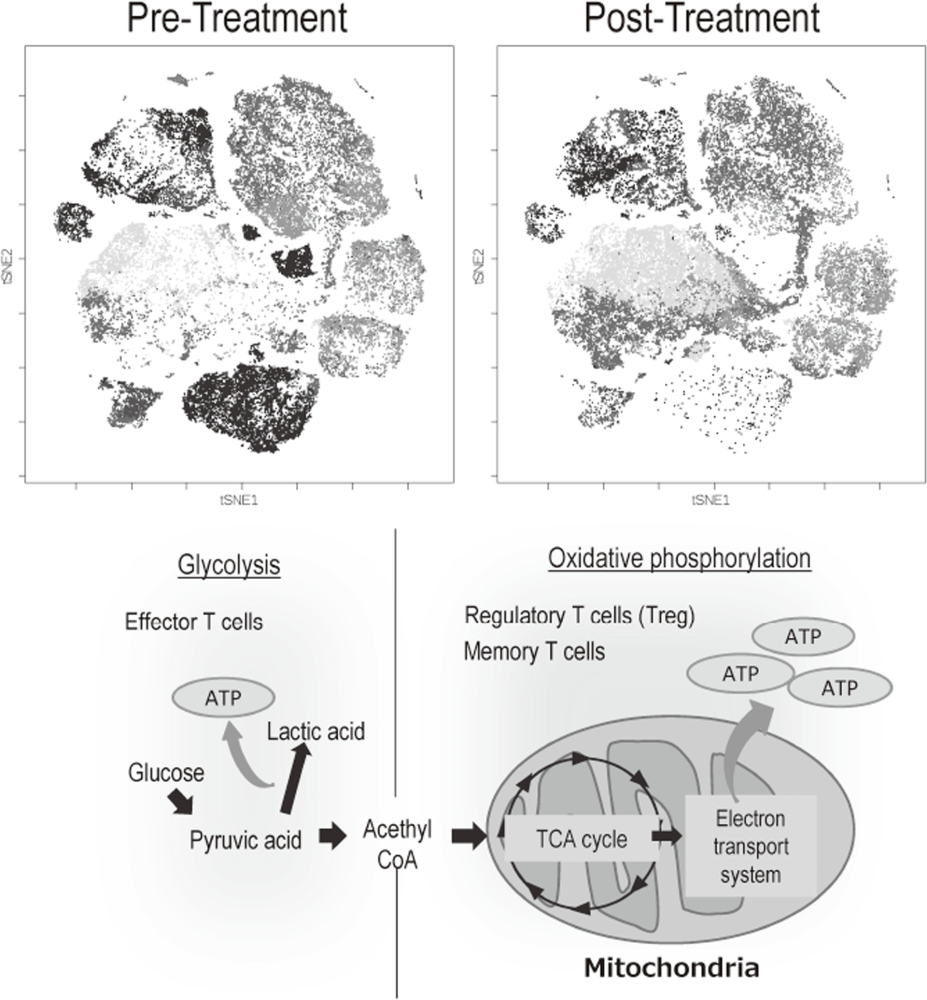
The Division of Cancer Immunology focused on elucidating the detailed mechanisms involved in tumor immune suppression or tumor immune evasion observed in various types of cancers. We have collected tissues and peripheral blood pre- and post-treatment with immune checkpoint inhibitors from many cancer patients. To comprehensively explore immune responses, molecular markers and gene expression profiles of TILs and PBMCs were examined. To this end, we established strategies to investigate multi-parameters with a single cell level using flow-cytometry and mass cytometry (CyTOF). Lympho-cytes are known to change their metabolism between glycolysis and oxidative phosphorylation according to their activity states and cell types such as effector T cells and regulatory T cells. We then interrogated the control mechanism of a metabolic shift of lymphocytes in immune suppression or immune evasion at tumor microenvironment (Figure 2).
Figure 2. Representative image of analysis of clinical-samples (pre- and post treatment), and metabolic pathway map in lymphocytes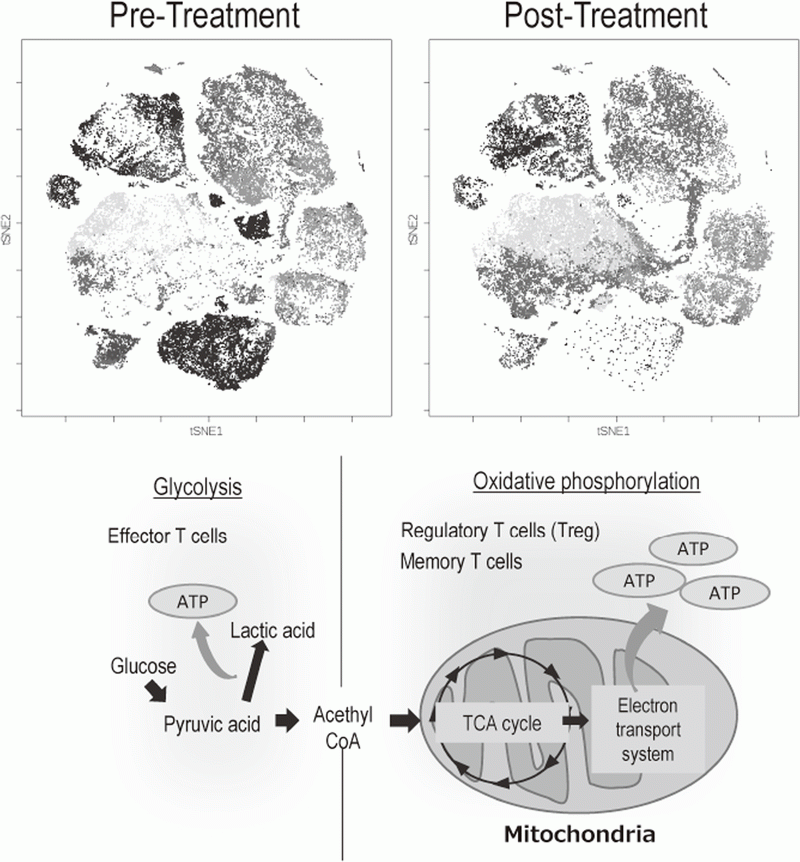
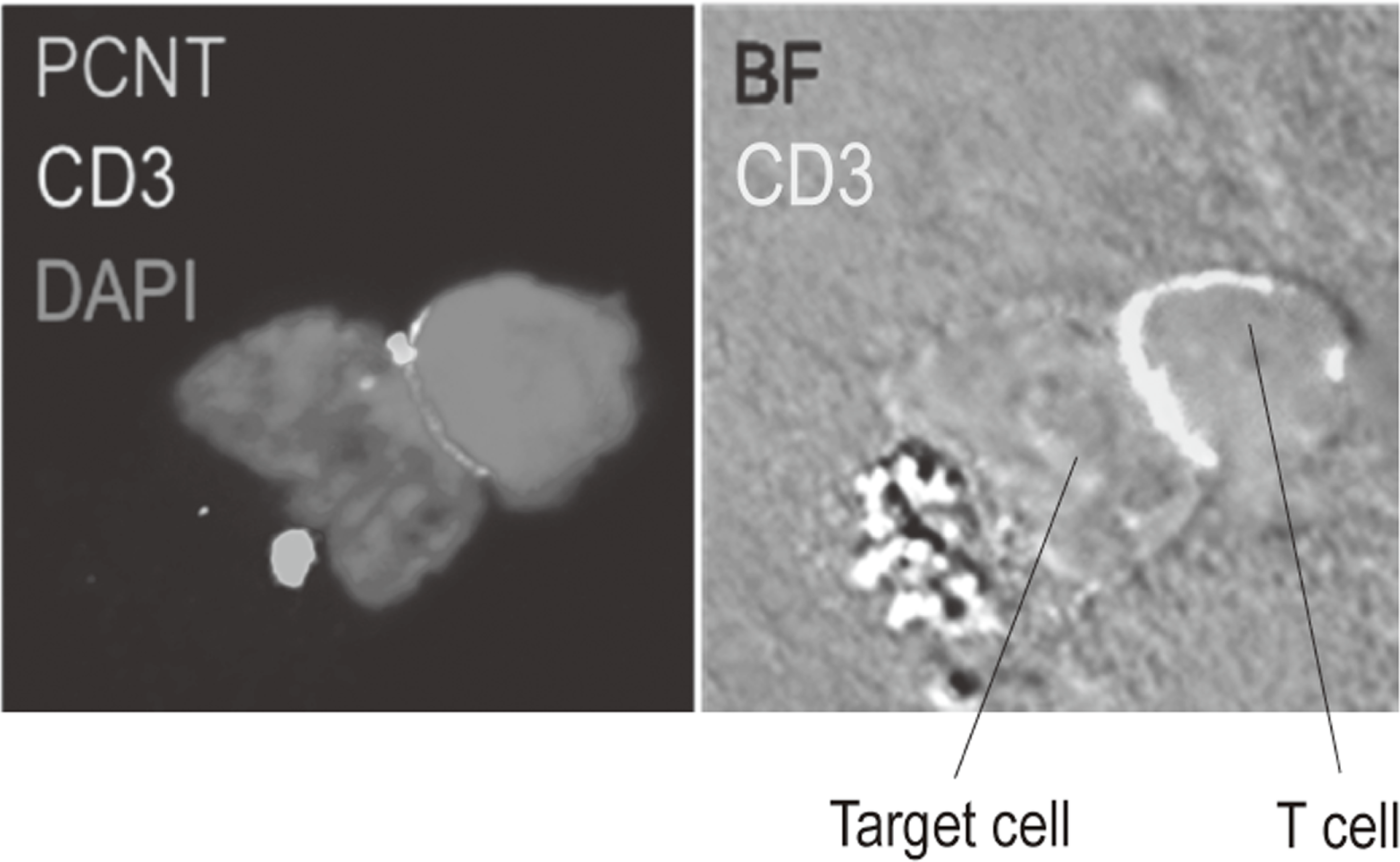
Additionally, to investigate anti-tumor immune responses in vivo, particularly focusing on tumor microenvironment, we developed mouse models and explored the pattern of the immunological phenotype according on the development/regression of tumor. Further, we also established a new method to evaluate the dynamics of molecular delivery within lymphocytes using a laser confocal microscopy (Figure 3).
Figure 3. Confocal microscopical image of molecular dynamics in lymphocytes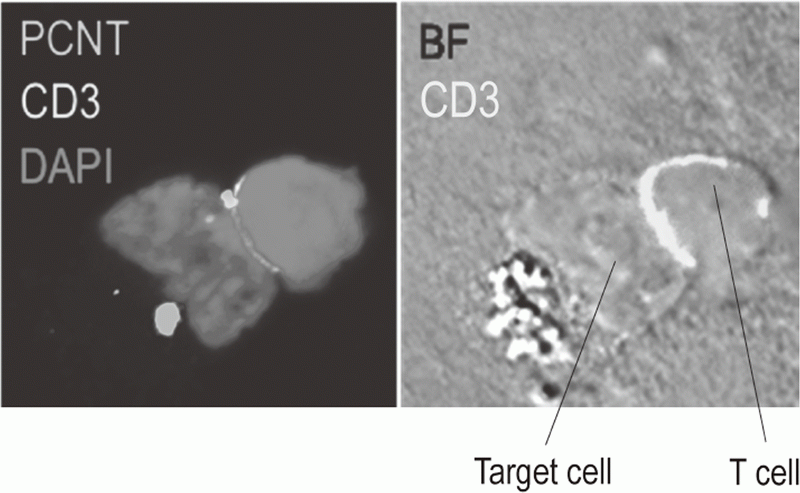
Education
Two graduate school students (Department of Dermatologic Oncology) and three post-doctoral fellows from various area are trained in our division. The combination of diverse fields is valuable to comprehensively understand cancer immunology. We actively promote recruitment of fellows and students from broad fields and collaborations with various fields such as genomics or data science.
Future prospects
Our division aims at elucidating the mechanisms involved in tumor immune suppression or tumor immune evasion in various types of cancers with a broad view such as immunology and genetics. In addition, we plan to investigate immune responses in more details by integrated analyses using not only immunological methods, but also single-cell based DNA/RNA seq, gene-editing and data science with humans and animal models. We pursue novel effective cancer immunotherapies and identify promising biomarkers based on the insights obtained from our fundamental research.
List of papers published in January 2017 - March 2018
Journal
1. Enokida T, Nishikawa H. Regulatory T cells, as a target in anticancer immunotherapy. Immunotherapy, 9:623-627, 2017
2. Togashi Y, Nishikawa H. Suppression from beyond the grave. Nat Immunol, 18:1285-1286, 2017
3. Doki N, Suyama M, Sasajima S, Ota J, Igarashi A, Mimura I, Morita H, Fujioka Y, Sugiyama D, Nishikawa H, Shimazu Y, Suda W, Takeshita K, Atarashi K, Hattori M, Sato E, Watakabe-Inamoto K, Yoshioka K, Najima Y, Kobayashi T, Kakihana K, Takahashi N, Sakamaki H, Honda K, Ohashi K. Clinical impact of pre-transplant gut microbial diversity on outcomes of allogeneic hematopoietic stem cell transplantation. Ann Hematol, 96:1517-1523, 2017
4. Kobayashi K, Suemasa F, Sagara H, Nakamura S, Ino Y, Hiramatsu H, Haraguchi T, Kurokawa K, Todo T, Nakano A, Iba H. MiR-199a Inhibits Secondary Envelopment of Herpes Simplex Virus-1 Through the Downregulation of Cdc42-specific GTPase Activating Protein Localized in Golgi Apparatus. Sci Rep, 7:6650, 2017
5. Hiramatsu H, Kobayashi K, Haraguchi T, Ino Y, Todo T, Iba H. The role of the SWI/SNF chromatin remodeling complex in maintaining the stemness of glioma initiating cells. Sci Rep, 7:889, 2017
6. Nishiwaki S, Sugiura I, Miyata Y, Saito S, Sawa M, Nishida T, Miyamura K, Kuwatsuka Y, Kohno A, Yuge M, Kasai M, Iida H, Kurahashi S, Osaki M, Goto T, Terakura S, Murata M, Nishikawa H, Kiyoi H. Efficacy and safety of autologous peripheral blood stem cell transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia: A study protocol for a multicenter exploratory prospective study (Auto-Ph17 study). Medicine (Baltimore), 96:e9568, 2017
7. Togashi Y, Nishikawa H. Regulatory T Cells: Molecular and Cellular Basis for Immunoregulation. Current topics in microbiology and immunology, 410:43186, 2017
8. Itoh T, Nishinakamura H, Kumano K, Takahashi H, Kodama S. The Spleen Is an Ideal Site for Inducing Transplanted Islet Graft Expansion in Mice. PLoS One, 12:e0170899, 2017
9. Kobayashi K, Hiramatsu H, Nakamura S, Haraguchi T, Iba H. Tumor suppression via inhibition of SWI/SNF complex-dependent NF-kappaB activation. Sci Rep, 7:11772, 2017
10. Nagase H, Takeoka T, Urakawa S, Morimoto-Okazawa A, Kawashima A, Iwahori K, Takiguchi S, Nishikawa H, Sato E, Sakaguchi S, Mori M, Doki Y, Wada H. ICOS+ Foxp3+ TILs in gastric cancer are prognostic markers and effector regulatory T cells associated with Helicobacter pylori. Int J Cancer, 140:686-695, 2017
11. Hamano Y, Kida H, Ihara S, Murakami A, Yanagawa M, Ueda K, Honda O, Tripathi LP, Arai T, Hirose M, Hamasaki T, Yano Y, Kimura T, Kato Y, Takamatsu H, Otsuka T, Minami T, Hirata H, Inoue K, Nagatomo I, Takeda Y, Mori M, Nishikawa H, Mizuguchi K, Kijima T, Kitaichi M, Tomiyama N, Inoue Y, Kumanogoh A. Classification of idiopathic interstitial pneumonias using anti-myxovirus resistance-protein 1 autoantibody. Sci Rep, 7:43201, 2017
12. Ueda T, Aokage K, Nishikawa H, Neri S, Nakamura H, Sugano M, Tane K, Miyoshi T, Kojima M, Fujii S, Kuwata T, Ochiai A, Kusumoto M, Suzuki K, Tsuboi M, Ishii G. Immunosuppressive tumor microenvironment of usual interstitial pneumonia-associated squamous cell carcinoma of the lung. J Cancer Res Clin Oncol, 144:835-844, 2018
13. Takeuchi Y, Tanemura A, Tada Y, Katayama I, Kumanogoh A, Nishikawa H. Clinical response to PD-1 blockade correlates with a sub-fraction of peripheral central memory CD4+ T cells in patients with malignant melanoma. Int Immunol, 30:13-22, 2018
14. Takeuchi Y, Tanemura A, Tada Y, Katayama I, Kumanogoh A, Nishikawa H. Clinical response to PD-1 blockade correlates with a sub-fraction of peripheral central memory CD4+ T cells in patients with malignant melanoma. Int Immunol, 30:13-22, 2018
