Annual Report 2017
Department of Cell Culture Technology
Tohru Kiyono, Ghani Farhana Ishrat, Chiho Kohno
Introduction
There are mainly two approaches to amplify cancer cells from patients, in vitro cell culture and patient-derived xenograft (PDX). Patient-derived xenografts (PDXs) generated from fresh tumor specimens generally reflect histopathology, tumor behavior, and the metastatic properties of the original tumor. However, PDX is generally costly and the success rate to establish new PDX lines is not satisfactory.
Our team and what we do
The Department of Cell Culture Technology was founded in 2014 for developing better methods to amplify normal human cells as well as cancer cells derived from clinical specimens obtained by operation, biopsy, and therapy. With the help of the Department of Pathology and Clinical Laboratories, we have started to store valuable cancer specimens systematically so that cancer tissues or cancer cells can be cultivated in vitro in the future.
Research activities
On the basis of the improved method developed by the Division of Carcinogenesis and Cancer Prevention, we now can cultivate so far difficult-to-cultivate primary human cells, such as hepatocytes, digestive tract epithelial cells, endometrial cells and oviduct epithelial cells. A method for a long-term culture of these cell types has been established, and they also can be immortalized by transduction of CDK4, cyclin D1 and TERT so as to be cultivated in more general culture conditions.
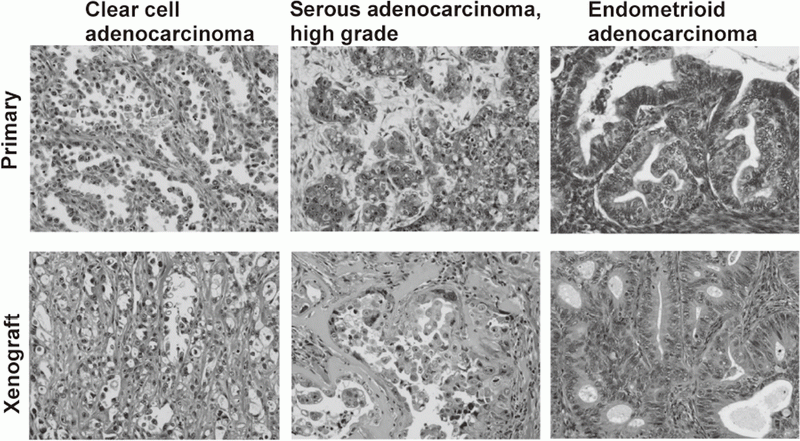
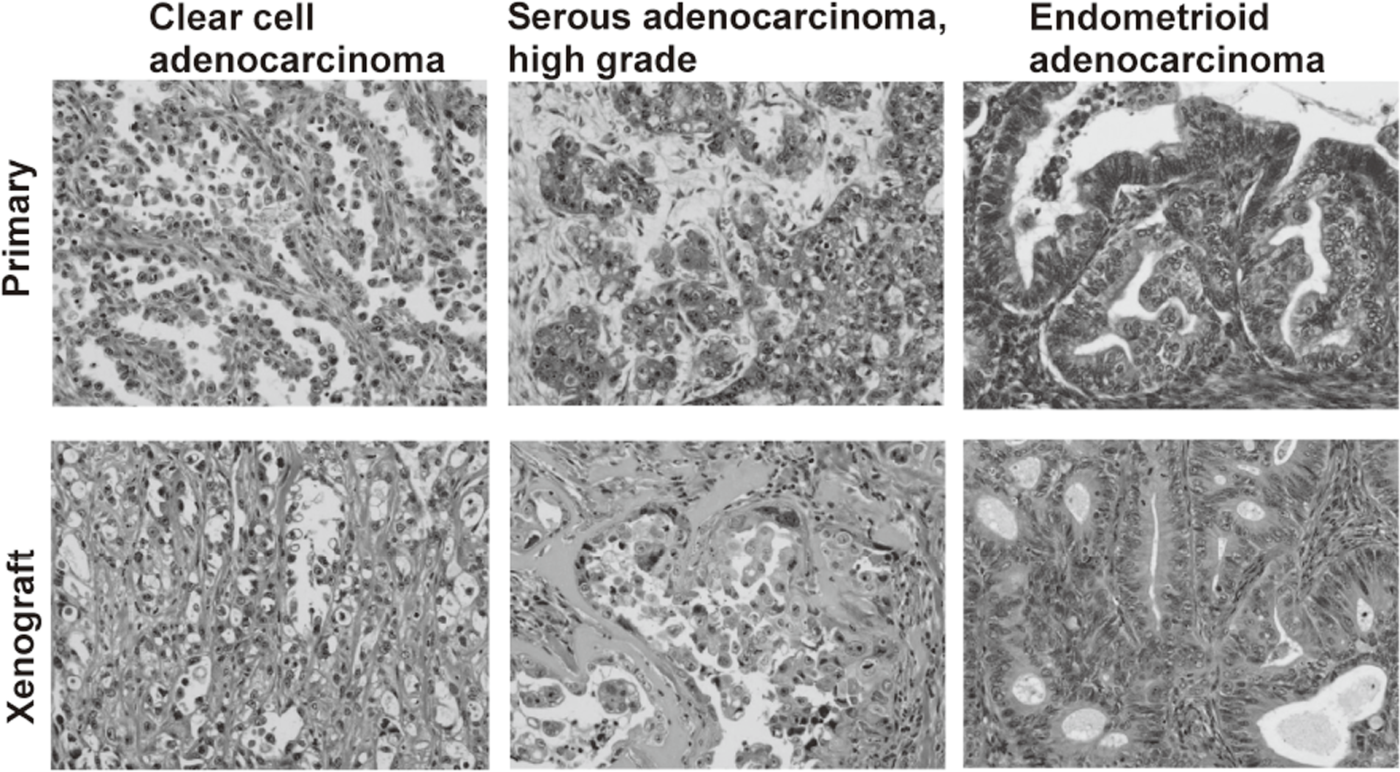
We have banked 23 new ovarian cancer specimens and reached over 80 in a deep freezer. Among them, we have established 19 ovarian cancer cell lines. Cell line-derived xenografts (CDX) were confirmed to faithfully recapitulate the features of original tumors. Some of the lines were not tumorigenic in nude mice, but conditional expression of additional oncogenes made them tumorigenic and the resultant tumor tissues after termination of the oncogene expression were confirmed to faithfully recapitulate the features of original tumors. Thus, we now are able to make xenograft tumors faithfully recapitulate the features of a given ovarian tumor.
Future prospects
Cancer cell lines established by ordinary culture methods generally do not maintain the features of original tumors. Additional mutations in p15/p16 genes and pathological features of xenograft tumors are often different from the original tumors. We have established a new culture method to amplify ovarian cancer cells at almost 100% efficiency. Xenograft tumors faithfully recapitulated the features of original tumors. On the basis of the improved culture methods of normal human epithelial cells, now we are trying to establish cell lines from precancerous lesions, such as endometriosis and cervical dysplasia. These cell lines are valuable resources with detailed clinical information. Systematic storage of live materials from valuable clinical samples is now under development.
List of papers published in January 2017 - March 2018
Journal
1. Noguchi K, Wakai K, Kiyono T, Kawabe M, Yoshikawa K, Hashimoto-Tamaoki T, Kishimoto H, Nakano Y. Molecular analysis of keratocystic odontogenic tumor cell lines derived from sporadic and basal cell nevus syndrome patients. Int J Oncol, 51:1731-1738, 2017
2. Hashimoto N, Kiyono T, Saitow F, Asada M, Yoshida M. Reversible differentiation of immortalized human bladder smooth muscle cells accompanied by actin bundle reorganization. PLoS One, 12:e0186584, 2017
3. Katayama M, Hirayama T, Kiyono T, Onuma M, Tani T, Takeda S, Nishimori K, Fukuda T. Immortalized prairie vole-derived fibroblasts (VMF-K4DTs) can be transformed into pluripotent stem cells and provide a useful tool with which to determine optimal reprogramming conditions. J Reprod Dev, 63:311-318, 2017
4. Shinohara M, Sumino Y, Sato F, Kiyono T, Hashimoto N, Mimata H. Tumor necrosis factor-alpha inhibits differentiation of myogenic cells in human urethral rhabdosphincter. Int J Urol, 24:461-467, 2017
5. Yamamoto E, Niimi K, Kiyono T, Yamamoto T, Nishino K, Nakamura K, Kotani T, Kajiyama H, Shibata K, Kikkawa F. Establishment and characterization of cell lines derived from complete hydatidiform mole. Int J Mol Med, 40:614-622, 2017
6. Hashimoto H, Suda Y, Miyashita T, Ochiai A, Tsuboi M, Masutomi K, Kiyono T, Ishii G. A novel method to generate single-cell-derived cancer-associated fibroblast clones. J Cancer Res Clin Oncol, 143:1409-1419, 2017
7. Kayama K, Watanabe S, Takafuji T, Tsuji T, Hironaka K, Matsumoto M, Nakayama KI, Enari M, Kohno T, Shiraishi K, Kiyono T, Yoshida K, Sugimoto N, Fujita M. GRWD1 negatively regulates p53 via the RPL11-MDM2 pathway and promotes tumorigenesis. EMBO Rep, 18:123-137, 2017
8. Fuchigami T, Koyama H, Kishida M, Nishizawa Y, Iijima M, Kibe T, Ueda M, Kiyono T, Maniwa Y, Nakamura N, Kishida S. Fibroblasts promote the collective invasion of ameloblastoma tumor cells in a 3D coculture model. FEBS open bio, 7:2000-2007, 2017
9. Nakamura K, Nakayama K, Ishikawa N, Ishikawa M, Sultana R, Kiyono T, Kyo S. Reconstitution of high-grade serous ovarian carcinoma from primary fallopian tube secretory epithelial cells. Oncotarget, 9:12609-12619, 2018
