Annual Report 2018
Division of Translational Genomics (Tsukiji Campus)
Takashi Kohno, Hitoshi Ichikawa, Takashi Kubo
Introduction
This division aims to facilitate cancer genome medicine by implementing nextgeneration sequencing (NGS)-based tumor profiling systems.
The Team and What We Do
This division organizes a clinical sequencing team with staff members from the Department of Experimental Therapeutics and Department of Pathology and Clinical Laboratories in the Hospital and aims to implement an NGS-based tumor profiling system called "NCC Oncopanel".
Research activities
1. Implementation of NCC Oncopanel clinical sequencing test
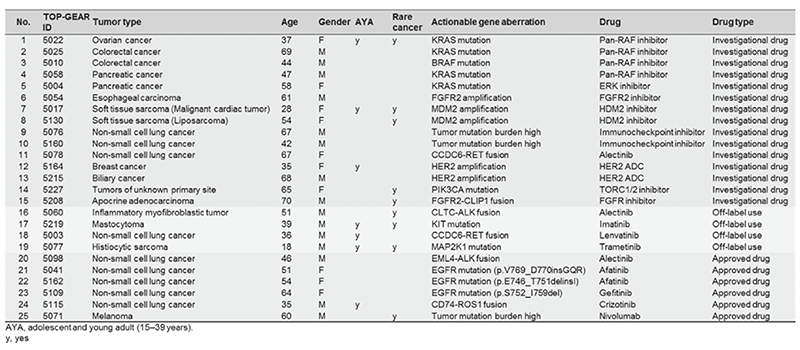
Next-generation sequencing (NGS) of tumor sample DNA (i.e. clinical sequencing) can guide clinical management by providing diagnostic or prognostic data and facilitating the identification of potential treatment regimens, such as molecular-targeted and immune checkpoint blockade therapies. However, no tumor-profiling gene panel tests have yet been implemented in routine oncological practice. By conducting a prospective cohort study (TOP-GEAR project; UMIN000011141), we have been implementing NGS-based gene-panel tests in routine oncological practice in Japan. We developed a NGS-based gene-panel test (NCC Oncopanel system) to examine somatic and germline mutations of 114 cancer-associated genes in advanced solid tumors. By using this system, we have shown that approximately 10% of examined cases have since received molecular-targeted therapy according to their gene aberrations (Table 1). The NCC Oncopanel system was approved by PMDA in the SAKIGAKE program of the MHLW (OncoGuide NCC Oncopanel System) as the first tumor-profiling gene panel tests in Japan on December 25, 2018 and will be reimbursed under the national insurance system in 2019.
2. Implementation of clinical sequencing in cancer genome medicine in Japan
In 2018, we validated the feasibility and clinical utility of the NCC oncopanel test within the Advanced Medical Care B system; the NCC Oncopanel test was tested by 50 Core or Liaison Hospitals for Cancer Genomic Medicine in Japan. This project also helped establish medical systems for clinical sequencing in many hospitals and would underpin cancer genome medicine in Japan.
Clinical trials
TOP-GEAR: Trial of Onco-Panel for Geneprofiling to Estimate both Adverse events and Response by cancer treatment (UMIN000011141)
Education
Post-doctoral fellows and chief residents in NCC were educated through "on the job training" in several translational research projects.
Future prospects
Tumor-profiling multiplex gene panel tests, including NCC Oncopanel, will underpin cancer genome medicine in Japan. However, at present, a gap exists between the number of patients with actionable mutations and those receiving genomically matched therapy. This gap is largely attributable to the lack of availability/accessibility of relevant trials and drugs. To fill it, we are collaborating with oncologists for the following three purposes: 1) To facilitate molecularly driven clinical trials by implementing tumor genome profiling tests, 2) To annotate VUSs (variants of unknown significance mutations) in druggable genes and 3) To establish clinical trials for gene alterations with a status changing from "undruggable" to "druggable", such as deleterious mutations in chromatin regulator genes.
List of papers published in 2018
Journal
1. Nakano Y, Tomiyama A, Kohno T, Yoshida A, Yamasaki K, Ozawa T, Fukuoka K, Fukushima H, Inoue T, Hara J, Sakamoto H, Ichimura K. Identification of a novel KLC1-ROS1 fusion in a case of pediatric low-grade localized glioma. Brain Tumor Pathol, 36:14-19, 2019
2. Ikemura S, Yasuda H, Matsumoto S, Kamada M, Hamamoto J, Masuzawa K, Kobayashi K, Manabe T, Arai D, Nakachi I, Kawada I, Ishioka K, Nakamura M, Namkoong H, Naoki K, Ono F, Araki M, Kanada R, Ma B, Hayashi Y, Mimaki S, Yoh K, Kobayashi SS, Kohno T, Okuno Y, Goto K, Tsuchihara K, Soejima K. Molecular dynamics simulation-guided drug sensitivity prediction for lung cancer with rare EGFR mutations. Proc Natl Acad Sci U S A, 116:10025-10030, 2019
3. Wirth LJ, Kohno T, Udagawa H, Ishii G, Ebata KB, Tuch B, Zhu EY, Nguyen M, Smith S, Hanson LM, Burkhard MR, Cable L, Blake JF, Condroski KR, Brandhuber BJ, Andrews S, Rothenberg SM, Goto K. Emergence and targeting of acquired and hereditary resistance to multikinase RET inhibition in RET-altered cancer patients. JCO Prec Oncol, 2019
4. Kuno I, Yoshida H, Kohno T, Ochiai A, Kato T. Endometrial cancer arising after complete remission of uterine malignant lymphoma: A case report and mutation analysis. Gynecol Oncol Rep, 28:50- 53, 2019
5. Sunami K, Ichikawa H, Kubo T, Kato M, Fujiwara Y, Shimomura A, Koyama T, Kakishima H, Kitami M, Matsushita H, Furukawa E, Narushima D, Nagai M, Taniguchi H, Motoi N, Sekine S, Maeshima A, Mori T, Watanabe R, Yoshida M, Yoshida A, Yoshida H, Satomi K, Sukeda A, Hashimoto T, Shimizu T, Iwasa S, Yon- List of papers published in 2018 Journal 454 emori K, Kato K, Morizane C, Ogawa C, Tanabe N, Sugano K, Hiraoka N, Tamura K, Yoshida T, Fujiwara Y, Ochiai A, Yamamoto N, Kohno T. Feasibility and utility of a panel testing for 114 cancer- associated genes in a clinical setting: A hospital-based study. Cancer Sci, 110:1480-1490, 2019
6. Kohno T. Implementation of "clinical sequencing" in cancer genome medicine in Japan. Cancer Sci, 109:507-512, 2018
7. Sereewattanawoot S, Suzuki A, Seki M, Sakamoto Y, Kohno T, Sugano S, Tsuchihara K, Suzuki Y. Identification of potential regulatory mutations using multi-omics analysis and haplotyping of lung adenocarcinoma cell lines. Sci Rep, 8:4926, 2018
8. Kashima Y, Suzuki A, Liu Y, Hosokawa M, Matsunaga H, Shirai M, Arikawa K, Sugano S, Kohno T, Takeyama H, Tsuchihara K, Suzuki Y. Combinatory use of distinct single-cell RNA-seq analytical platforms reveals the heterogeneous transcriptome response. Sci Rep, 8:3482, 2018
9. Itahashi K, Kondo S, Kubo T, Fujiwara Y, Kato M, Ichikawa H, Koyama T, Tokumasu R, Xu J, Huettner CS, Michelini VV, Parida L, Kohno T, Yamamoto N. Evaluating Clinical Genome Sequence Analysis by Watson for Genomics. Front Med, 5:305, 2018
10. Kato M, Nakamura H, Nagai M, Kubo T, Elzawahry A, Totoki Y, Tanabe Y, Furukawa E, Miyamoto J, Sakamoto H, Matsumoto S, Sunami K, Arai Y, Suzuki Y, Yoshida T, Tsuchihara K, Tamura K, Yamamoto N, Ichikawa H, Kohno T, Shibata T. A computational tool to detect DNA alterations tailored to formalin-fixed paraffin- embedded samples in cancer clinical sequencing. Genome Med, 10:44, 2018
