Annual Report 2018
Department of Head and Neck Medical Oncology
Makoto Tahara, Susumu Okano, Takao Fujisawa, Yuri Ueda, Kazue Ito
Introduction
The Head and Neck Medical Oncology Department is engaged in the clinical management of patients with head and neck cancer (HNC), and research into anticancer drugs for the treatment of HNC.
Our missions are to: 1) provide the best evidence-based treatment; 2) promote the importance of supportive care in the treatment of patients with HNC; 3) facilitate the timely approval of new drugs by active participation in global clinical trials to eliminate the drug lag; 4) develop cutting-edge treatments; and 5) train experts in head and neck medical oncology.
The Team and What We Do
Our department consists of three physicians, one senior resident and one resident. We manage the treatment of HNC patients who receive anticancer drugs. An estimated 60% of HNC patients require a multidisciplinary approach, including surgery, radiotherapy, and chemotherapy. Given the increasing complexity of the management of HNC, recommended treatment for patients who are referred to our institution is decided at a weekly tumor board attended by a multidisciplinary team.
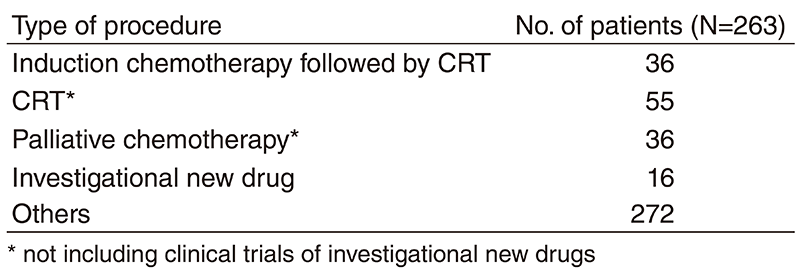
A total of 415 patients were referred to our department from April 2018 to March 2019 (Table 1, Table 2). The outpatient service of our department is available from Monday to Friday. We carefully follow patients during and after treatment and provide palliative chemotherapy as an outpatient service.
Table 1. Number of patients according to sites
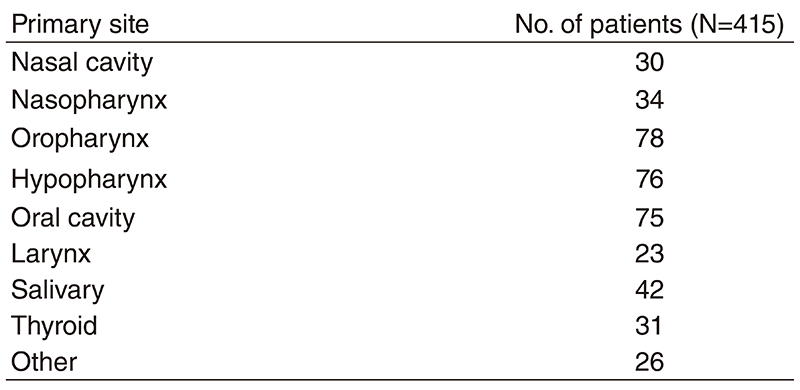

Table 2. No. of patients according to procedure

Research activities
Our research activity has focused on three areas, the development of new treatments in clinical trials for HNC, biomarker analysis in HNC and retrospective analysis of management of treatment for HNC.
We evaluate the feasibility of TPF as induction chemotherapy (IC) for Japanese patients and the tolerability of CRT with fractionated administration of cisplatin after IC (Okano S, International Journal of Clinical Oncology 2019). Forty-eight patients were enrolled. The IC treatment completion rate was 85.4%. Grade 3-4 toxicities of TPF were neutropenia (79.2%) and febrile neutropenia (14.6%). Thirty-eight patients (79.2%) achieved a response after IC. Forty patients subsequently underwent CRT and four received radiation alone. Thirty-three patients (82.5%) completed the planned cycles of fractionated administration of cisplatin. With a median follow-up of 36.1 months, 3-year OS was 65.0%. TPF IC is feasible and subsequent CRT with fractionated administration of cisplatin is tolerable.
In a phase II study, we assessed the feasibility of docetaxel, cisplatin, and cetuximab (TPEx) followed by cetuximab and concurrent radiotherapy for LA SCCHN (Zenda S, Front Oncology 2019). We enrolled 54 patients. The overall response rate was 72.2% with a median follow-up of 36.1 months and a 3-year overall survival of 90.7%. The treatment completion rate was 76%. The frequencies of grade ≥three febrile neutropenia or allergy/infusion reactions were 39% and 11%, respectively. IC with TPEx followed by cetuximab with concurrent radiotherapy showed acceptable compliance for the treatment of LA SCCHN. However, high frequency of febrile neutropenia remains a challenge and further improvement in the management of TPEx is necessary.
In the phase 3 SELECT, lenvatinib significantly improved efficacy outcomes vs placebo in patients with radioiodine-refractory differentiated thyroid cancer (RR-DTC). This exploratory, post hoc analysis investigated the impact of dose interruption on lenvatinib efficacy (Tahara M, Eur Journal of Cancer, 2019). In a multivariate model, dose interruption was significantly associated with lenvatinib efficacy, even after adjustment for patient characteristics. Patients with shorter dose interruptions (<10% of total treatment duration) had a greater magnitude of benefit vs those with longer interruptions (≥10%). This analysis highlights the importance of timely management of lenvatinib toxicities to minimize dose interruptions and maximize lenvatinib efficacy in patients with RR-DTC.
Clinical trials
The following investigator-initiated clinical trials are ongoing; 1) JCOG1008: a randomized phase 2/3 trial of postoperative CRT comparing weekly CDDP plus RT with three weekly CDDP plus RT in high risk patients with SCCHN, 2) a phase 2 study of lenvatinib for anaplastic thyroid cancer and 3) a cohort study exploring the effect of lenvatinib on differentiated thyroid cancer 4) a phase 2 study of a combination with nivolumab plus lenvatinib for unresectable anaplastic thyroid cancer.
To facilitate the timely approval of new drugs and eliminate the drug lag, we have also participated in the global phase trials including immune-checkpoint inhibitors.
Education
We teach not only medical staff in our institute but also outside of our institute by conducting the following education programs: 1) Seminar of the Japan Society of Supportive Care for Patients with HNC and 2) Preceptorship in HNC. Furthermore, our department is accepting trainees all the time.
Future prospects
We hope that ongoing or planned clinical trials will change the standard of care for HNC and our biomarker analysis will lead to the development of new treatment strategy. Our education program will increase the number of medical oncologists who take charge of treatment for HNC, leading to improving patient's quality of life.
List of papers published in 2018
Journal
1. Matsuzuka T, Kiyota N, Mizusawa J, Akimoto T, Fujii M, Hasegawa Y, Iwae S, Monden N, Matsuura K, Onozawa Y, Hayashi R, Tahara M. Clinical impact of cachexia in unresectable locally advanced head and neck cancer: supplementary analysis of a phase II trial (JCOG0706-S2). Jpn J Clin Oncol, 49:37-41, 2019
2. Tahara M, Brose MS, Wirth LJ, Suzuki T, Miyagishi H, Fujino K, Dutcus CE, Gianoukakis A. Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine-refractory differentiated thyroid cancer. Eur J Cancer, 106:61-68, 2019
3. Okano S, Enokida T, Onoe T, Ota Y, Motegi A, Zenda S, Akimoto T, Tahara M. Induction TPF chemotherapy followed by CRT with fractionated administration of cisplatin in patients with unresectable locally advanced head and neck cancer. Int J Clin Oncol, 2019
4. Takahashi S, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, Fukuda N, Sasaki T, Suzuki T, Ikezawa H, Dutcus CE, Tahara M. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol, 15:717-726, 2019
5. Zenda S, Ota Y, Kiyota N, Okano S, Fujii M, Kitamura M, Takahashi S, Ueda T, Monden N, Yamanaka T, Tahara M. A Multicenter Phase II Trial of Docetaxel, Cisplatin, and Cetuximab (TPEx) Followed by Cetuximab and Concurrent Radiotherapy for Patients With Local Advanced Squamous Cell Carcinoma of the Head and Neck (CSPOR HN01: ECRIPS Study). Front Oncol, 9:6, 2019
6. Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V, Adelstein D, Van Gestel D, Vermorken JB. Low-Dose vs HighDose Cisplatin: Lessons Learned From 59 Chemoradiotherapy Trials in Head and Neck Cancer. Front Oncol, 9:86, 2019
7. Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V, Adelstein D, Vermorken JB. Altered fractionation radiotherapy combined with concurrent low-dose or high-dose cisplatin in head and neck cancer: A systematic review of literature and meta-analysis. Oral oncology, 76:52-60, 2018
8. . Tahara M, Muro K, Hasegawa Y, Chung HC, Lin CC, Keam B, Takahashi K, Cheng JD, Bang YJ. Pembrolizumab in Asia-Pacific patients with advanced head and neck squamous cell carcinoma: Analyses from KEYNOTE-012. Cancer Sci, 109:771-776, 2018
9. Tahara M. Management of recurrent or metastatic thyroid cancer. ESMO open, 3:e000359, 2018
10. .Gillison ML, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Li L, Ferris RL. CheckMate 141: 1-Year Update and Subgroup Analysis of Nivolumab as First-Line Therapy in Patients with Recurrent/Metastatic Head and Neck Cancer. Oncologist, 23:1079-1082, 2018
11. Tahara M, Kiyota N, Yokota T, Hasegawa Y, Muro K, Takahashi S, Onoe T, Homma A, Taguchi J, Suzuki M, Minato K, Yane K, Ueda S, Hara H, Saijo K, Yamanaka T. Phase II trial of combination treatment with paclitaxel, carboplatin and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CSPOR-HN02). Ann Oncol, 29:1004-1009, 2018
12. Tahara M, Takami H, Ito Y, Sugino K, Takahashi S, Takeyama H, Tsutsui H, Hara H, Mitsuma A, Yamashita H, Okamoto T, Sugitani I, Ohashi Y, Imai T. Cohort study exploring the effect of lenvatinib on differentiated thyroid cancer. Endocr J, 65:1071-1074, 2018
13. Wirth LJ, Tahara M, Robinson B, Francis S, Brose MS, Habra MA, Newbold K, Kiyota N, Dutcus CE, Mathias E, Guo M, Sherman SI, Schlumberger M. Treatment-emergent hypertension and efficacy in the phase 3 Study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer, 124:2365-2372, 2018
14. Uozumi S, Enokida T, Suzuki S, Nishizawa A, Kamata H, Okano T, Fujisawa T, Ueda Y, Okano S, Tahara M, Yamaguchi M. Predictive Value of Cetuximab-Induced Skin Toxicity in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and NECK. Front Oncol, 8:616, 2018
15. Enokida T, Okano S, Fujisawa T, Ueda Y, Uozumi S, Tahara M. Paclitaxel Plus Cetuximab as 1st Line Chemotherapy in Platinum-Based Chemoradiotherapy-Refractory Patients With Squamous Cell Carcinoma of the Head and Neck. Front Oncol, 8:339, 2018
Book
1. Enokida T, Okano S, Tahara M. section IV Recurrentor Metastaitic Disease 23 Recurrent or Metastatic HNSCC: Systemic Therapy. In: Argiris A, Ferris RL, Rosenthal DI (ed), Head and Neck Cancers: Evidence-Based Treatment 1st Edition, USA, Demos Medical, pp 423-446, 2018
