Annual Report 2018
Department of Breast and Medical Oncology
Toru Mukohara, Hirofumi Mukai, Ako Hosono, Yoichi Naito, Nobuaki Matsubara, Takahiro Kogawa, Kenichi Harano, Yumi Fujimoto, Shota Kusuhara, Yoriko Hasegawa
Introduction
In the clinic, we are taking care of patients with breast, urologic, gynecologic, primaryunknown cancers, and sarcoma. We are also in charge of rare cancers that are difficult to be treated at other hospitals because of the absence of standard therapy.
We contributed to developing the "Lady's center" that opened in 2018 as a core of multidisciplinary care for women with cancer. We also played central roles in developing institutional infrastructure for multi-gene panel testing, which will be launched under insurance coverage. Further, we ran the Hereditary Breast and Ovarian Cancer syndrome (HBOC) clinic to provide proper information to patients with a high risk of the syndrome and to act as a bridge to Genetic Medicine and Services.
Research activities
We are implementing retrospective and prospective clinical studies as well as translational studies. We recently initiated a collaborative study with industry utilizing their technology for detecting circulating tumor cells (CTCs).
We are also implementing clinical and translational research with funds obtained from Grant-in-Aid for Scientific Research ("3D coculture of cancer cells in malignant effusion/ CTC with adipose stem cell"; PI, Mukohara T) ("New sub-classification of triple negative breast cancer to evaluate immunogenicity"; PI, Kogawa T). This year, we ran a preclinical project about combination therapy for HER2-positive breast cancer in collaboration with the Division of Genome TR.
Education
Our goal in education is fostering "genuine" medical oncologists. Graduates from our Resident/Chief resident programs are expected to be capable of providing not only standard therapies regardless of types of cancer but also multi-disciplinary care cooperating with other medical professionals. Obtaining skills for palliative care and dealing with oncologic emergencies are also expected. Further, we require them and help them to implement clinical research to address clinical questions they find for themselves and report the results on internal journals. That is because we want to develop scientific clinicians who can focus on issues scientifically and create evidence for themselves. This year, one graduate from our residency program earned Diplomate, Subspecialty Board of Medical Oncology from JSMO.
Further, we were awarded an educational grant with the theme "Comprehensive educational program to develop medical professionals and peer supporters to empower Adolescent and Young Adult (AYA) breast cancer patients". We are implementing the program from next year.
Future prospects
In the clinic, we will provide clinical care with high patient satisfaction through a multidisciplinary team approach. In education, we will foster medical oncologists who have skill and knowledge in cross-organ oncology care and scientific acumen. In research, we will initiate multi-center clinical trials and phase I to III developmental trials of investigational drugs. We will also expand our research activity to preclinical research for response predictions and overcoming resistance for anti-cancer drugs. Multiple research themes are currently under discussion with industries and other academic organizations.
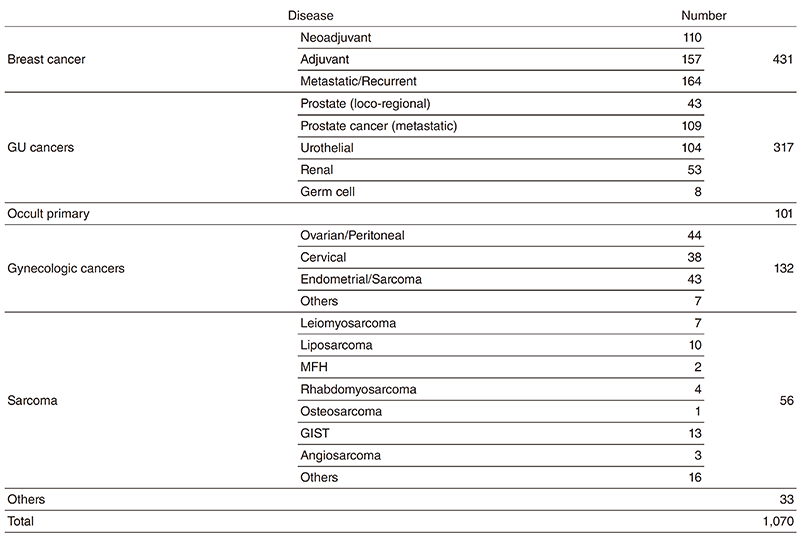
Table 1. Number of patients (April, 2018 - March, 2019)

List of papers published in 2018
Journal
1. Esaki T, Hirai F, Makiyama A, Seto T, Bando H, Naito Y, Yoh K, Ishihara K, Kakizume T, Natsume K, Myers A, Doi T. Phase I dose-escalation study of capmatinib (INC280) in Japanese patients with advanced solid tumors. Cancer Sci, 110:1340-1351, 2019
2. Uemura H, Uemura H, Nagamori S, Wakumoto Y, Kimura G, Kikukawa H, Yokomizo A, Mizokami A, Kosaka T, Masumori N, Kawasaki Y, Yonese J, Nasu Y, Fukasawa S, Sugiyama T, Kinuya S, Hosono M, Yamaguchi I, Akagawa T, Matsubara N. Threeyear follow-up of a phase II study of radium-223 dichloride in Japanese patients with symptomatic castration-resistant prostate cancer and bone metastases. Int J Clin Oncol, 24:557-566, 2019
3. Chayahara N, Mukohara T, Tachihara M, Fujishima Y, Fukunaga A, Washio K, Yamamoto M, Nakata K, Kobayashi K, Takenaka K, Toyoda M, Kiyota N, Tobimatsu K, Doi H, Mizuta N, Marugami N, Kawaguchi A, Nishigori C, Nishimura Y, Minami H. Adapalene Gel 01% Versus Placebo as Prophylaxis for Anti-Epidermal Growth Factor Receptor-Induced Acne-Like Rash: A Randomized Left-Right Comparative Evaluation (APPEARANCE). Oncologist, 2019
4. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulieres D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med, 380:1116-1127, 2019
5. Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, Jievaltas M, Luz M, Alekseev B, Kuss I, Kappeler C, Snapir A, Sarapohja T, Smith MR. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med, 380:1235-1246, 2019
6. Smith M, Parker C, Saad F, Miller K, Tombal B, Ng QS, Boegemann M, Matveev V, Piulats JM, Zucca LE, Karyakin O, Kimura G, Matsubara N, Nahas WC, Nole F, Rosenbaum E, Heidenreich A, Kakehi Y, Zhang A, Krissel H, Teufel M, Shen J, Wagner V, Higano C. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol, 20:408-419, 2019
7. Hayashi H, Kurata T, Takiguchi Y, Arai M, Takeda K, Akiyoshi K, Matsumoto K, Onoe T, Mukai H, Matsubara N, Minami H, Toyoda M, Onozawa Y, Ono A, Fujita Y, Sakai K, Koh Y, Takeuchi A, Ohashi Y, Nishio K, Nakagawa K. Randomized Phase II Trial Comparing Site-Specific Treatment Based on Gene Expression Profiling With Carboplatin and Paclitaxel for Patients With Cancer of Unknown Primary Site. J Clin Oncol, 37:570-579, 2019
8. Mukai H, Shimizu C, Masuda N, Ohtani S, Ohno S, Takahashi M, Yamamoto Y, Nishimura R, Sato N, Ohsumi S, Iwata H, Mori Y, Hashigaki S, Muramatsu Y, Nagasawa T, Umeyama Y, Lu DR, Toi M. Palbociclib in combination with letrozole in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-2 subgroup analysis of Japanese patients. Int J Clin Oncol, 24:274-287, 2019
9. Goto H, Shimono Y, Funakoshi Y, Imamura Y, Toyoda M, Kiyota N, Kono S, Takao S, Mukohara T, Minami H. Adipose-derived stem cells enhance human breast cancer growth and cancer stem cell-like properties through adipsin. Oncogene, 38:767-779, 2019
10. Miura Y, Imamura CK, Uchino K, Kishida T, Matsubara N, Shinojima T, Kondo K, Hongo F, Yoshimura K, Tanigawara Y, Takano T. Individualized Dosing of Axitinib Based on First-Dose Area Under the Concentration-Time Curve for Metastatic Renal-Cell Carcinoma. Clin Genitourin Cancer, 17:e1-e11, 2019
11. 0. Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet, 393:1440-1452, 2019
12. Kumano Y, Hasegawa Y, Kawahara T, Yasui M, Miyoshi Y, Matsubara N, Uemura H. Pretreatment Neutrophil to Lymphocyte Ratio (NLR) Predicts Prognosis for Castration Resistant Prostate Cancer Patients Underwent Enzalutamide. Biomed Res Int, 2019:9450838, 2019
13. Shimokawa M, Hayashi T, Kogawa T, Matsui R, Mizuno M, Kikkawa F, Saeki T, Aiba K, Tamura K. Evaluation of Combination Antiemetic Therapy on CINV in Patients With Gynecologic Cancer Receiving TC Chemotherapy. Anticancer Res, 39:225-230, 2019
14. Yonemori K, Shimomura A, Yasojima H, Masuda N, Aogi K, Takahashi M, Naito Y, Shimizu S, Nakamura R, Hashimoto J, Yamamoto H, Hirakawa A, Michimae H, Hamada A, Yoshida T, Sukigara T, Tamura K, Fujiwara Y. A phase I/II trial of olaparib tablet in combination with eribulin in Japanese patients with advanced or metastatic triple-negative breast cancer previously treated with anthracyclines and taxanes. Eur J Cancer, 109:84-91, 2019
15. Fujiwara Y, Mukai H, Saeki T, Ro J, Lin YC, Nagai SE, Lee KS, Watanabe J, Ohtani S, Kim SB, Kuroi K, Tsugawa K, Tokuda Y, Iwata H, Park YH, Yang Y, Nambu Y. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br J Cancer, 120:475-480, 2019
16. Doi T, Yang JC, Shitara K, Naito Y, Cheng AL, Sarashina A, Pronk LC, Takeuchi Y, Lin CC. Phase I Study of the Focal Adhesion Kinase Inhibitor BI 853520 in Japanese and Taiwanese Patients with Advanced or Metastatic Solid Tumors. Target Oncol, 14:57- 65, 2019
17. Ohashi Y, Ikeda M, Kunitoh H, Sasako M, Okusaka T, Mukai H, Fujiwara K, Nakamura M, Kimura T, Ibusuki K, Sakon M. Venous thromboembolism in patients with cancer: design and rationale of a multicentre, prospective registry (Cancer-VTE Registry). BMJ Open, 8:e018910, 2018
18. Goto H, Kiyota N, Otsuki N, Imamura Y, Chayahara N, Suto H, Nagatani Y, Toyoda M, Mukohara T, Nibu KI, Kasahara T, Ito Y, Miya A, Hirokawa M, Miyauchi A, Minami H. Successful treatment switch from lenvatinib to sorafenib in a patient with radioactive iodine-refractory differentiated thyroid cancer intolerant to lenvatinib due to severe proteinuria. Auris Nasus Larynx, 45:1249-1252, 2018
19. Fukasawa S, Suzuki H, Kawaguchi K, Noguchi H, Enjo K, Tran N, Todd M, Fizazi K, Matsubara N. Efficacy and safety of abiraterone acetate plus prednisone in Japanese patients with newly diagnosed, metastatic hormone-naive prostate cancer: a subgroup analysis of LATITUDE, a randomized, double-blind, placebo-controlled, Phase 3 study. Jpn J Clin Oncol, 48:1012- 1021, 2018
20. Matsubara N, Naito Y, Nakano K, Fujiwara Y, Ikezawa H, Yusa W, Namiki M, Okude T, Takahashi S. Lenvatinib in combination with everolimus in patients with advanced or metastatic renal cell carcinoma: A phase 1 study. Int J Urol, 25:922-928, 2018
21. Harano K, Wang Y, Lim B, Seitz RS, Morris SW, Bailey DB, Hout DR, Skelton RL, Ring BZ, Masuda H, Rao AUK, Laere SV, Bertucci F, Woodward WA, Reuben JM, Krishnamurthy S, Ueno NT. Rates of immune cell infiltration in patients with triple-negative breast cancer by molecular subtype. PLoS ONE, 13:e0204513, 2018
22. Kono M, Fujii T, Matsuda N, Harano K, Chen H, Wathoo C, Joon AY, Tripathy D, Meric-Bernstam F, Ueno NT. Somatic mutations, clinicopathologic characteristics, and survival in patients with untreated breast cancer with bone-only and non-bone sites of first metastasis. J Cancer, 9:3640-3646, 2018
23. Kogawa T, Fujii T, Fouad TM, Liu DD, Harano K, Masuda H, Iwase T, Barnett C, Park YS, Lim B, Tripathy D, Litton JK, Ueno NT. Impact of change in body mass index during neoadjuvant chemotherapy and survival among breast cancer subtypes. Breast Cancer Res Treat, 171:501-511, 2018
24. Iwamoto T, Taira N, Fujisawa T, Araki K, Sakamaki K, Sangai T, Kikawa Y, Shien T, Takao S, Sato M, Goto Y, Yoshida T, Takahashi M, Aihara T, Mukai H. Hormonal Therapy Resistant Estrogen-receptor Positive Metastatic Breast Cancer Cohort (HORSE-BC) Study : Current Status of Treatment Selection in Japan. Acta Med Okayama, 72:369-374, 2018
25. Ding Q, Wang Y, Zuo Z, Gong Y, Krishnamurthy S, Li CW, Lai YJ, Wei W, Wang J, Manyam GC, Diao L, Zhang X, Lin F, Symmans WF, Sun L, Liu CG, Liu X, Debeb BG, Ueno NT, Harano K, Alvarez RH, Wu Y, Cristofanilli M, Huo L. Decreased expression of microRNA-26b in locally advanced and inflammatory breast cancer. Hum Pathol, 77:121-129, 2018
26. Naito Y, Urasaki T. Precision medicine in breast cancer. Chin Clin Oncol, 7:29, 2018
27. Mukai H, Ito M. Advances in chemotherapy for HER2-negative metastatic breast cancer. Chin Clin Oncol, 7:26, 2018
28. Tatara T, Mukohara T, Tanaka R, Shimono Y, Funakoshi Y, Imamura Y, Toyoda M, Kiyota N, Hirai M, Kakeji Y, Minami H. 3D Culture Represents Apoptosis Induced by Trastuzumab Better than 2D Monolayer Culture. Anticancer Res, 38:2831-2839, 2018
29. Tatara T, Mukohara T, Shimono Y, Yamasaki T, Imamura Y, Funakoshi Y, Toyoda M, Kiyota N, Takao S, Kono S, Kakeji Y, Minami H. Expression of programmed death-1 in sentinel lymph nodes of breast cancer. J Surg Oncol, 117:1131-1136, 2018
30. Yasui M, Hasegawa Y, Kawahara T, Kumano Y, Miyoshi Y, Matsubara N, Uemura H. Baseline neutrophil-to-lymphocyte ratio predicts the prognosis of castration-resistant prostate cancer treated with abiraterone acetate. Mol Clin Oncol, 8:592-594, 2018
31. Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H, Lopez-Gitlitz A, Trudel GC, Espina BM, Shu Y, Park YC, Rackoff WR, Yu MK, Small EJ. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med, 378:1408- 1418, 2018
32. Matsubara N, Yamada Y, Tabata KI, Satoh T, Kamiya N, Suzuki H, Kawahara T, Uemura H, Yano A, Kawakami S, Otsuka M, Fukasawa S. Abiraterone Followed by Enzalutamide Versus Enzalutamide Followed by Abiraterone in Chemotherapy-naive Patients With Metastatic Castration-resistant Prostate Cancer. Clin Genitourin Cancer, 16:142-148, 2018
33. Yamaguchi T, Mukai H, Takahashi M, Hara F, Yamauchi C, Yamashita S, Ushijima T. Predictive value of genetic analysis for pathological complete response to preoperative treatment in HER2 positive, HR negative early breast cancer (PASSION trial). Jpn J Clin Oncol, 48:388-391, 2018
34. Iwasa S, Yamamoto N, Shitara K, Tamura K, Matsubara N, Tajimi M, Lin AB, Asou H, Cai Z, Inoue K, Shibasaki Y, Saito K, Takai H, Doi T. Dose-finding study of the checkpoint kinase 1 inhibitor, prexasertib, in Japanese patients with advanced solid tumors. Cancer Sci, 109:3216-3223, 2018
35. Naito Y, Takahashi H, Shitara K, Okamoto W, Bando H, Kuwata T, Kuboki Y, Matsumoto S, Miki I, Yamanaka T, Watanabe A, Kojima M. Feasibility study of cancer genome alterations identified by next generation sequencing: ABC study. Jpn J Clin Oncol, 48:559-564, 2018
36. Sunami K, Takahashi H, Tsuchihara K, Takeda M, Suzuki T, Naito Y, Sakai K, Dosaka-Akita H, Ishioka C, Kodera Y, Muto M, Wakai T, Yamazaki K, Yasui W, Bando H, Fujimoto Y, Fukuoka S, Harano K, Kawazoe A, Kimura G, Koganemaru S, Kogawa T, Kotani D, Kuboki Y, Matsumoto H, Matsumoto S, Mishima S, Nakamura Y, Sawada K, Shingaki S, Shitara K, Umemoto K, Umemura S, Yasuda K, Yoshino T, Yamamoto N, Nishio K. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (Edition 10). Cancer Sci, 109:2980-2985, 2018
37. Nishimura M, Hayashi M, Mizutani Y, Takenaka K, Imamura Y, Chayahara N, Toyoda M, Kiyota N, Mukohara T, Aikawa H, Fujiwara Y, Hamada A, Minami H. Distribution of erlotinib in rash and normal skin in cancer patients receiving erlotinib visualized by matrix assisted laser desorption/ionization mass spectrometry imaging. Oncotarget, 9:18540-18547, 2018
38. Ishihara H, Yamashita S, Fujii S, Tanabe K, Mukai H, Ushijima T. DNA methylation marker to estimate the breast cancer cell fraction in DNA samples. Med Oncol, 35:147, 2018
39. .Komai Y, Gotohda N, Matsubara N, Takeda H, Yuasa T, Inoue M, Yamamoto S, Yonese J. Preliminary Kidney Parenchymal Ligation Using Endoloop Ligatures-A Simple Method to Achieve a Trifecta in Laparoscopic Partial Nephrectomy Without Hilar Clamping for Polar Complex Tumors. Urology, 121:182-188, 2018
