Annual Report 2018
Department of General Internal Medicine
Toshihiko Doi, Keiji Okinaka, Shigeki Umemura, Takahiro Kogawa, Yusuke Hashimoto, Yasutoshi Kuboki, Tomohumi Miura, Nobuhiko Yamauchi
Introduction
1. General internal medicine
We aim to develop a collaborative team within the department of internal medicine whereby physicians treat patients with systemic therapy (i.e. chemotherapy, targeting agent, hormone therapy and immune checkpoint inhibitor etc.) so that we can improve patient safety by controlling treatment-related adverse events (AEs).
2. Infectious diseases
The mission of the infectious diseases section is to provide consultation on clinical infectious diseases. We also work with the Office of Infection Control and Prevention to prevent healthcare-associated infections during cancer care.
The Team and What We Do
1. General internal medicine
We focus on the supportive consultation for drug-related adverse events (AEs), particularly immune-checkpoint inhibitor agents. We sometimes experience unexpected immunerelated AEs and this consultation system helps in solving and recovering from severe AEs. Further, our group member has outstanding insights into each form of cancer treatment and we collectively facilitate collaborative treatment and research.
2. Infectious diseases
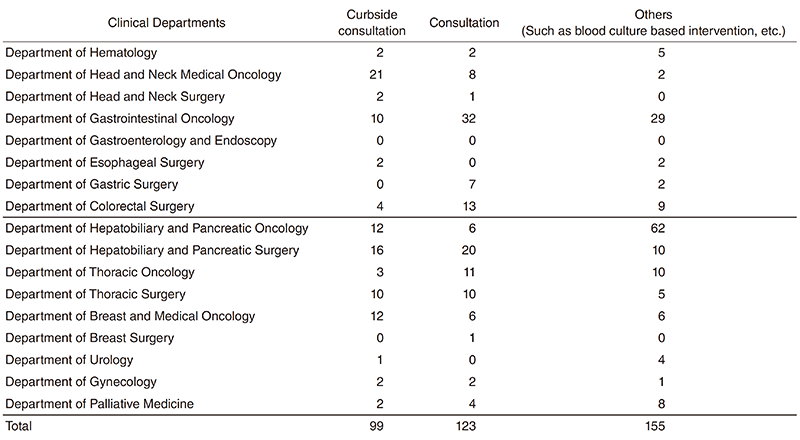
We provided 377 infectious disease consultations in this period (Table 1) and also promote hospital infection control (See "Office of Infection Control and Prevention section").
The number of cases managed during this period:
- Positive blood culture cases: 341
- Cases using broad-spectrum antibiotics: 1,211
Table 1. Number of consultations

Research activities
1. General internal medicine
We are performing research into immunerelated adverse events in our institution.
2. Infectious diseases None.
Clinical trials
1. General internal medicine None.
2. Infectious diseases None.
Education
1. General internal medicine
Each staff member teaches cancer treatment and patient management through bedside learning and conference. We also strive to ensure trainees have opportunities to present at annual conferences and write manuscripts. If indicated, trainees will have the opportunity to prepare clinical research LOIs and protocols.
2. Infectious diseases
We have provided lectures on infectious diseases for the benefit of trainees. Okinaka also implemented an antimicrobial stewardship program at the Department of Hematopoietic Stem Cell Transplantation of the National Cancer Center Hospital.
We were approved as one of the training facilities for the Japanese Association for Infectious Diseases in July 2018.
Future prospects
1. General internal medicine
We further collaborate with regional institutions to assemble outstanding trainees and will nurture close connections with several huge institutions. We prepare a safety treatment manual for systemic therapy-related AE and develop safe and cost-effective practice. We plan to have approximately 150 case consultations of immune-related AEs.
2. Infectious Diseases
Consultation services for Infectious diseases are now increasingly recognized as key components of cancer centers, some of which have begun to establish a department for infectious diseases. The future goal is to launch fellowship programs for fellows to develop highlevel expertise and assume a key role in this field.
List of papers published in 2018
Journal
1. Miura T, Matsumoto Y, Kawaguchi T, Masuda Y, Okizaki A, Koga H, Tagami K, Watanabe YS, Uehara Y, Yamaguchi T, Morita T. Low Phase Angle Is Correlated With Worse General Condition in Patients with Advanced Cancer. Nutr Cancer, 71:83-88, 2019
2. Shimokawa M, Hayashi T, Kogawa T, Matsui R, Mizuno M, Kikkawa F, Saeki T, Aiba K, Tamura K. Evaluation of Combination Antiemetic Therapy on CINV in Patients With Gynecologic Cancer Receiving TC Chemotherapy. Anticancer Res, 39:225-230, 2019
3. Mehnert JM, Varga A, Brose MS, Aggarwal RR, Lin CC, Prawira A, de Braud F, Tamura K, Doi T, Piha-Paul SA, Gilbert J, Saraf S, Thanigaimani P, Cheng JD, Keam B. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer, 19:196, 2019
4. Kawazoe A, Shitara K, Kuboki Y, Bando H, Kojima T, Yoshino T, Ohtsu A, Ochiai A, Togashi Y, Nishikawa H, Doi T, Kuwata T. Clinicopathological features of 22C3 PD-L1 expression with mismatch repair, Epstein-Barr virus status, and cancer genome alterations in metastatic gastric cancer. Gastric Cancer, 22:69- 76, 2019
5. Mishima S, Kawazoe A, Nakamura Y, Sasaki A, Kotani D, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, Kuwata T, Tsuji A, Shitara K. Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J Immunother Cancer, 7:24, 2019
6. Doi T, Yang JC, Shitara K, Naito Y, Cheng AL, Sarashina A, Pronk LC, Takeuchi Y, Lin CC. Phase I Study of the Focal Adhesion Kinase Inhibitor BI 853520 in Japanese and Taiwanese Patients with Advanced or Metastatic Solid Tumors. Target Oncol, 14:57- 65, 2019
7. Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, Rugo HS, Cohen RB, O'Neil BH, Mehnert JM, Lopez J, Doi T, van Brummelen EMJ, Cristescu R, Yang P, Emancipator K, Stein K, Ayers M, Joe AK, Lunceford JK. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. J Clin Oncol, 37:318-327, 2019
8. Shimomura A, Yamamoto N, Kondo S, Fujiwara Y, Suzuki S, Yanagitani N, Horiike A, Kitazono S, Ohyanagi F, Doi T, Kuboki Y, Kawazoe A, Shitara K, Ohno I, Banerji U, Sundar R, Ohkubo S, Calleja EM, Nishio M. First-in-Human Phase I Study of an Oral HSP90 Inhibitor, TAS-116, in Patients with Advanced Solid Tumors. Mol Cancer Ther, 18:531-540, 2019
9. Imaoka H, Sasaki M, Takahashi H, Hashimoto Y, Ohno I, Mitsunaga S, Watanabe K, Umemoto K, Kimura G, Suzuki Y, Kan M, Ikeda M. Alternate Endpoints for Phase II Trials in Advanced Neuroendocrine Tumors. Oncologist, 24:47-53, 2019
10. Ikeda M, Ohno I, Ueno H, Mitsunaga S, Hashimoto Y, Okusaka T, Kondo S, Sasaki M, Sakamoto Y, Takahashi H, Hara R, Kobayashi S, Nakamura O, Morizane C. Phase I study of resminostat, an HDAC inhibitor, combined with S-1 in patients with pre-treated biliary tract or pancreatic cancer. Invest New Drugs, 37:109-117, 2019
11. Suzuki Y, Kan M, Kimura G, Umemoto K, Watanabe K, Sasaki M, Takahashi H, Hashimoto Y, Imaoka H, Ohno I, Mitsunaga S, Ikeda M. Predictive factors of the treatment outcome in patients with advanced biliary tract cancer receiving gemcitabine plus cisplatin as first-line chemotherapy. J Gastroenterol, 54:281-290, 2019
12. Hashimoto Y, Ohno I, Takahashi H, Sasaki M, Imaoka H, Watanabe K, Umemoto K, Kimura G, Mitsunaga S, Ikeda M. EUS-guided n-butyl-2-cyanoacrylate injection therapy for ruptured isolated left gastric artery pseudoaneurysm. Endosc Ultrasound, 8:58-59, 2019
13. Watanabe YS, Miura T, Okizaki A, Tagami K, Matsumoto Y, Fujimori M, Morita T, Kinoshita H. Comparison of Indicators for Achievement of Pain Control With a Personalized Pain Goal in a Comprehensive Cancer Center. J Pain Symptom Manage, 55:1159-1164, 2018
14. Kako J, Kobayashi M, Kanno Y, Ogawa A, Miura T, Matsumoto Y. The Optimal Cutoff Point for Expressing Revised Edmonton Symptom Assessment System Scores as Binary Data Indicating the Presence or Absence of Symptoms. Am J Hosp Palliat Care, 35:1390-1393, 2018
15. Tagami K, Okizaki A, Miura T, Watanabe YS, Matsumoto Y, Morita T, Fujimori M, Kinoshita H. Breakthrough Cancer Pain Influences General Activities and Pain Management: A Comparison of Patients with and without Breakthrough Cancer Pain. J Palliat Med, 21:1636-1640, 2018
16. Miura T, Amano K, Shirado A, Baba M, Ozawa T, Nakajima N, Suga A, Matsumoto Y, Shimizu M, Shimoyama S, Kuriyama T, Matsuda Y, Iwashita T, Mori I, Kinoshita H. Low Transthyretin Levels Predict Poor Prognosis in Cancer Patients in Palliative Care Settings. Nutr Cancer, 70:1283-1289, 2018
17. Sawaki A, Yamada Y, Yamaguchi K, Nishina T, Doi T, Satoh T, Chin K, Boku N, Omuro Y, Komatsu Y, Hamamoto Y, Koizumi W, Saji S, Shah MA, Van Cutsem E, Kang YK, Iwasaki J, Kuriki H, Ohtsuka W, Ohtsu A. Regional differences in advanced gastric cancer: exploratory analyses of the AVAGAST placebo arm. Gastric Cancer, 21:429-438, 2018
18. Kogawa T, Fujii T, Fouad TM, Liu DD, Harano K, Masuda H, Iwase T, Barnett C, Park YS, Lim B, Tripathy D, Litton JK, Ueno NT. Impact of change in body mass index during neoadjuvant chemotherapy and survival among breast cancer subtypes. Breast Cancer Res Treat, 171:501-511, 2018
19. Matsumoto H, Sasaki A, Nakamura Y, Kawazoe A, Kuboki Y, Okinaka K, Shitara K. Tuberculous Meningitis during Chemotherapy for Advanced Gastric Cancer. Case reports in oncology, 11:228-233, 2018
20. Kuboki Y, Schatz CA, Koechert K, Schubert S, Feng J, Wittemer-Rump S, Ziegelbauer K, Krahn T, Nagatsuma AK, Ochiai A. In situ analysis of FGFR2 mRNA and comparison with FGFR2 gene copy number by dual-color in situ hybridization in a large cohort of gastric cancer patients. Gastric Cancer, 21:401-412, 2018
21. Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, Alsina M, Ghidini M, Faustino C, Gorbunova V, Zhavrid E, Nishikawa K, Hosokawa A, Yalcin S, Fujitani K, Beretta GD, Van Cutsem E, Winkler RE, Makris L, Ilson DH, Tabernero J. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol, 19:1437-1448, 2018
22. Mishima S, Kawazoe A, Matsumoto H, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, Nonte EM, Chintharlapalli S, Nasir A, Kuwata T, Shitara K. Efficacy and safety of ramucirumab-containing chemotherapy in patients with pretreated metastatic gastric neuroendocrine carcinoma. ESMO open, 3:e000443, 2018
23. Segal NH, He AR, Doi T, Levy R, Bhatia S, Pishvaian MJ, Cesari R, Chen Y, Davis CB, Huang B, Thall AD, Gopal AK. Phase I Study of Single-Agent Utomilumab (PF-05082566), a 4-1BB/ CD137 Agonist, in Patients with Advanced Cancer. Clin Cancer Res, 24:1816-1823, 2018
24. Bang YJ, Takano T, Lin CC, Fasanmade A, Yang H, Danaee H, Asato T, Kalebic T, Wang H, Doi T. TAK-264 (MLN0264) in Previously Treated Asian Patients with Advanced Gastrointestinal Carcinoma Expressing Guanylyl Cyclase C: Results from an Open-Label, Non-randomized Phase 1 Study. Cancer Res Treat, 50:398-404, 2018
25. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA oncology, 4:e180013, 2018
26. Nishina T, Takahashi S, Iwasawa R, Noguchi H, Aoki M, Doi T. Safety, pharmacokinetic, and pharmacodynamics of erdafitinib, a pan-fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitor, in patients with advanced or refractory solid tumors. Invest New Drugs, 36:424-434, 2018
27. Naito Y, Takahashi H, Shitara K, Okamoto W, Bando H, Kuwata T, Kuboki Y, Matsumoto S, Miki I, Yamanaka T, Watanabe A, Kojima M. Feasibility study of cancer genome alterations identified by next generation sequencing: ABC study. Jpn J Clin Oncol, 48:559-564, 2018
28. Sunami K, Takahashi H, Tsuchihara K, Takeda M, Suzuki T, Naito Y, Sakai K, Dosaka-Akita H, Ishioka C, Kodera Y, Muto M, Wakai T, Yamazaki K, Yasui W, Bando H, Fujimoto Y, Fukuoka S, Harano K, Kawazoe A, Kimura G, Koganemaru S, Kogawa T, Kotani D, Kuboki Y, Matsumoto H, Matsumoto S, Mishima S, Nakamura Y, Sawada K, Shingaki S, Shitara K, Umemoto K, Umemura S, Yasuda K, Yoshino T, Yamamoto N, Nishio K. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (Edition 10). Cancer Sci, 109:2980-2985, 2018
29. Tada Y, Togashi Y, Kotani D, Kuwata T, Sato E, Kawazoe A, Doi T, Wada H, Nishikawa H, Shitara K. Targeting VEGFR2 with Ramucirumab strongly impacts effector/ activated regulatory T cells and CD8+ T cells in the tumor microenvironment. J Immunother Cancer, 6:106, 2018
30. Miura T, Mitsunaga S, Ikeda M, Ohno I, Takahashi H, Kuwata T, Ochiai A. Neural Invasion Spreads Macrophage-Related Allodynia via Neural Root in Pancreatic Cancer. Anesth Analg, 126:1729- 1738, 2018
31. Miura T, Mitsunaga S, Ikeda M, Ohno I, Takahashi H, Suzuki H, Irisawa A, Kuwata T, Ochiai A. Characterization of low active ghrelin ratio in patients with advanced pancreatic cancer. Support Care Cancer, 26:3811-3817, 2018
32. Suzuki Y, Hashimoto Y, Shibuki T, Kan M, Kimura G, Umemoto K, Watanabe K, Sasaki M, Takahashi H, Imaoka H, Ohno I, Mitsunaga S, Ikeda M. Endoscopic Ultrasound-Guided Gallbladder Drainage for Aberrant Right Posterior Duct Obstruction Developing after Placement of a Covered Self-Expandable Metallic Stent in a Patient with Distal Biliary Obstruction. Case Rep Gastroenterol, 12:722-728, 2018
