Annual Report 2018
Department of Breast and Medical Oncology
Kenji Tamura, Kan Yonemori, Emi Noguchi, Akihiko Shimomura, Kazuki Sudo, Maki Tanioka, Hitomi Okuma, Tadaaki Nishikawa, Takuji Seo, Yohei Ohtake, Yuki Kojima, Yasuhiro Fujiwara
Introduction
The Department of Breast and Medical Oncology provides the most effective treatments by the use of chemotherapy, and works on the establishment of new standards of care for adult malignancies including breast cancer, gynecologic cancer, soft-tissue sarcoma, and extragonadal germ cell tumor, cancer of unknown primary and other rare types of solid tumors.
We envision becoming a leading medical oncology department, which makes a difference in cancer care in Japan and in the world. Our mission is to provide patient-centered, state-ofthe-art medical care to cancer patients, to develop new effective cancer treatments through clinical and translational research, and to nurture medical oncologists. An evidence-based, research-oriented and multi-disciplinary approach is the core value of our practice.
The Team and What We Do
1. Setup
Our division consists of eight full-time attending physicians, four chief residents (fellows), and two to three clinical residents. We also provide educational opportunities to shortterm (half-year) residents. Full-time attending physicians are on duty at the outpatient clinic two to three days per week. Inpatient management is undertaken by clinical teams, which consist of attending physicians and residents. A 'Grand Round' is scheduled every Wednesday and Friday.
2. Performance
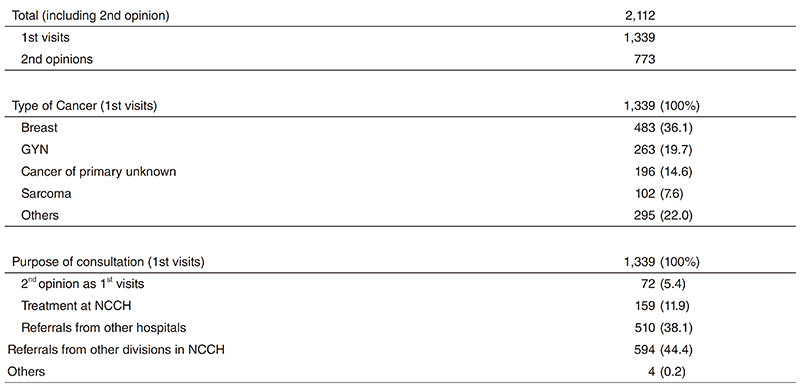
There were 2,112 first visits of new patients in 2018 (Table 1). A total of 36.1% of new patients are breast cancer patients, 19.7% are gynecological, 14.6% are primary unknown and 7.6% are soft-tissue sarcoma in the first visits. We did 773 second opinions in 2018.
Table 1. 1st Visiting Patients to the Department of Breast and Medical Oncology (2018)

3. Conference
The one-hour briefing medical conferences are held every morning to discuss the evidencedbased care for individual patients. The Phase 1 conference is held on Monday, Journal Club on Wednesday, Clinical trial conference on Thursday, and the Weekend and Outpatient follow-up conference on Friday. Multidisciplinary Case Conferences with diagnostic radiologists, surgeons, and pathologists are held with members of the department of Breast Surgery, Gynecology, Musculoskeletal Oncology and Rehabilitation, Radiation Oncology and Pathology, each once or twice (Breast) per week, respectively.
The Monthly Breast Cancer Conference is held with the participation of the multidisciplinary specialists to discuss recent topics in breast oncology and to update institutional treatment guidelines. This year, we published "Nyugan-shinnryou Application Notebook" from Nankodo based on these guidelines, which reflects the consensus of the breast team on the body of evidence on breast cancer management.
Research activities
Our research interest extends across a wide range of topics related to treatment and clinical program development. Many of our researches are secured by public and consignment research grants. In 2018, we conducted many research programs as a primary investigator and participated in additional programs as a coinvestigator in research programs secured by competitive public research funds. We published 44 international manuscripts, focusing on early phase anti-cancer drug development, molecular imaging, and translational research, novel chemotherapy against sarcoma and ovarian cancer, novel biomarkers to predict efficacy and adverse events of anti-cancer drugs and other basic research. We value cancer survivorship as a research theme in order to develop a comprehensive patient-centered care program.
Clinical trials
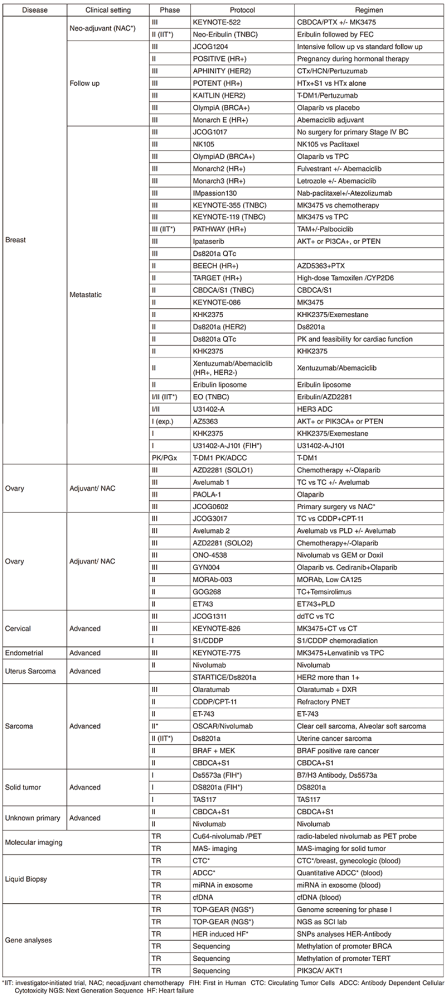
In 2018, we actively enrolled patients in phase I studies (including first in human or global) as well as domestic and international phase II and III studies (Table 2). Of note we launched a pharmacokinetic and dose-finding study of eribulin/olaparib, a phase II study of eribulin in a neoadjuvant setting for patients with triple negative breast cancer, a phase III study of tamoxifen with / without palbociclib, Ds8201a for uterine cancer sarcoma and a phase I of RPN2 (first in human) as investigator-initiated clinical trials (IIT in Table 2). New molecular imaging studies are launched in cooperation with research institutes. We also conducted many types of translational studies (TR) to find novel biomarkers.
Education
We provide rich educational opportunities to both residents and chief residents through clinical experience as well as research activities. Residents are encouraged to make presentations at local and national conferences. We vigorously support basic, clinical, or translational research conducted by postdoctoral researchers.
Future prospects
We will continue to establish new standard treatments and propose a near-future model of clinical management of adult solid tumors, including breast cancer, and gynecologic cancer. Moreover, we aim to build a comprehensive program, which includes tumor registry, translational research, clinical trials and patient care in rare adult tumors based on our rich clinical experience. We would also like to improve the efficiency of anti-cancer drug development by coordinating basic and translational research in early-phase clinical trials.
Table 2. Active Clinical Trials (2018)

List of papers published in 2018
Journal
1.Hirakawa A, Sudo K, Yonemori K, Sadachi R, Kinoshita F, Kobayashi Y, Okuma HS, Kawachi A, Tamura K, Fujiwara Y, Rubinstein L, Takebe N. A Comparative Study of Longitudinal Toxicities of Cytotoxic Drugs, Molecularly Targeted Agents, Immunomodulatory Drugs, and Cancer Vaccines. Clin Pharmacol Ther, 2019
2.Takahashi K, Yunokawa M, Sasada S, Takehara Y, Miyasaka N, Kato T, Tamura K. A novel prediction score for predicting the baseline risk of recurrence of stage I-II endometrial carcinoma. J Gynecol Oncol, 30:e8, 2019
3.Noguchi E, Tamura K, Hattori M, Horiguchi J, Sato N, Kanatani K, Matsunaga K, Iwata H, Fujiwara Y. Trastuzumab emtansine plus pertuzumab in Japanese patients with HER2-positive metastatic breast cancer: a phase Ib study. Breast Cancer, 26:39-46, 2019
4.Tanaka R, Yonemori K, Hirakawa A, Kinoshita F, Kobayashi Y, Yamazaki N, Fujimoto M, Tamura K, Fujiwara Y. Anticancer Agent-Induced Life-Threatening Skin Toxicities: A Database Study of Spontaneous Reporting Data. Oncologist, 24:266-272, 2019
5.Turner NC, Alarcon E, Armstrong AC, Philco M, Lopez Chuken YA, Sablin MP, Tamura K, Gomez Villanueva A, Perez-Fidalgo JA, Cheung SYA, Corcoran C, Cullberg M, Davies BR, de Bruin EC, Foxley A, Lindemann JPO, Maudsley R, Moschetta M, Outhwaite E, Pass M, Rugman P, Schiavon G, Oliveira M. BEECH: A dose-finding run-in followed by a randomised phase 2 study assessing the efficacy of AKT inhibitor capivasertib (AZD5363) combined with paclitaxel in patients with oestrogen receptor-positive advanced or metastatic breast cancer, and in a PIK3CA mutant sub-population. Ann Oncol, 2019
6.Mehnert JM, Varga A, Brose MS, Aggarwal RR, Lin CC, Prawira A, de Braud F, Tamura K, Doi T, Piha-Paul SA, Gilbert J, Saraf S, Thanigaimani P, Cheng JD, Keam B. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer, 19:196, 2019
7.Sunami K, Ichikawa H, Kubo T, Kato M, Fujiwara Y, Shimomura A, Koyama T, Kakishima H, Kitami M, Matsushita H, Furukawa E, Narushima D, Nagai M, Taniguchi H, Motoi N, Sekine S, Maeshima A, Mori T, Watanabe R, Yoshida M, Yoshida A, Yoshida H, Satomi K, Sukeda A, Hashimoto T, Shimizu T, Iwasa S, Yonemori K, Kato K, Morizane C, Ogawa C, Tanabe N, Sugano K, Hiraoka N, Tamura K, Yoshida T, Fujiwara Y, Ochiai A, Yamamoto N, Kohno T. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci, 110:1480-1490, 2019
8.Noda-Narita S, Shimomura A, Kawachi A, Sumiyoshi-Okuma H, Sudo K, Shimoi T, Noguchi E, Yonemori K, Shimizu C, Fujiwara Y, Tamura K. Comparison of the efficacy of trastuzumab emtansine between patients with metastatic human epidermal growth factor receptor 2-positive breast cancers previously treated with combination trastuzumab and pertuzumab and with trastuzumab only in Japanese population. Breast Cancer, 2019
9.Sato J, Shimoi T, Shimomura A, Noguchi E, Kodaira M, Yunokawa M, Yonemori K, Shimizu C, Fujiwara Y, Yoshida M, Tamura K. The Incidence of Nonmalignant Diseases among Patients with Suspected Carcinoma of Unknown Primary Site. Intern Med, 58:1423-1428, 2019
10.Yonemori K, Shimomura A, Yasojima H, Masuda N, Aogi K, Takahashi M, Naito Y, Shimizu S, Nakamura R, Hashimoto J, Yamamoto H, Hirakawa A, Michimae H, Hamada A, Yoshida T, Sukigara T, Tamura K, Fujiwara Y. A phase I/II trial of olaparib tablet in combination with eribulin in Japanese patients with advanced or metastatic triple-negative breast cancer previously treated with anthracyclines and taxanes. Eur J Cancer, 109:84-91, 2019
11.Fujiwara Y, Mukai H, Saeki T, Ro J, Lin YC, Nagai SE, Lee KS, Watanabe J, Ohtani S, Kim SB, Kuroi K, Tsugawa K, Tokuda Y, Iwata H, Park YH, Yang Y, Nambu Y. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br J Cancer, 120:475-480, 2019
12.Wong KY, Fan C, Tanioka M, Parker JS, Nobel AB, Zeng D, Lin DY, Perou CM. I-Boost: an integrative boosting approach for predicting survival time with multiple genomics platforms. Genome Biol, 20:52, 2019
13.Hironaka-Mitsuhashi A, Tsuda H, Yoshida M, Shimizu C, Asaga S, Hojo T, Tamura K, Kinoshita T, Ushijima T, Hiraoka N, Fujiwara Y. Invasive breast cancers in adolescent and young adult women show more aggressive immunohistochemical and clinical features than those in women aged 40-44 years. Breast Cancer, 26:386-396, 2019
14.Shiino S, Matsuzaki J, Shimomura A, Kawauchi J, Takizawa S, Sakamoto H, Aoki Y, Yoshida M, Tamura K, Kato K, Kinoshita T, Kitagawa Y, Ochiya T. Serum miRNA-based Prediction of Axillary Lymph Node Metastasis in Breast Cancer. Clin Cancer Res, 25:1817-1827, 2019
15.Adams S, Loi S, Toppmeyer D, Cescon DW, De Laurentiis M, Nanda R, Winer EP, Mukai H, Tamura K, Armstrong A, Liu MC, Iwata H, Ryvo L, Wimberger P, Rugo HS, Tan AR, Jia L, Ding Y, Karantza V, Schmid P. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol, 30:405-411, 2019
16.Yonemori K, Kodaira M, Satoh T, Kudo T, Takahashi S, Nakano K, Ando Y, Shimokata T, Mori J, Inoue K, Oakley GJ, Sakaguchi S, Tamura K. Phase 1 study of olaratumab plus doxorubicin in Japanese patients with advanced soft-tissue sarcoma. Cancer Sci, 109:3962-3970, 2018
17.Sawada T, Hilhorst R, Rangarajan S, Yoshida M, Tanabe Y, Tamura K, Kinoshita T, Shimoyama T, van Beuningen R, Ruijtenbeek R, Tsuda H, Koizumi F. Inactive immune pathways in triple negative breast cancers that showed resistance to neoadjuvant chemotherapy as inferred from kinase activity profiles. Oncotarget, 9:34229-34239, 2018
18.Iwasa S, Yamamoto N, Shitara K, Tamura K, Matsubara N, Tajimi M, Lin AB, Asou H, Cai Z, Inoue K, Shibasaki Y, Saito K, Takai H, Doi T. Dose-finding study of the checkpoint kinase 1 inhibitor, prexasertib, in Japanese patients with advanced solid tumors. Cancer Sci, 109:3216-3223, 2018
19.Sawaki M, Tamura K, Shimomura A, Taki Y, Nagashima F, Iwata H. Editors' Choice Practice management for elderly patients with breast cancer; Findings from a survey by the Japan Breast Cancer Study Group. Nagoya J Med Sci, 80:217-226, 2018
20.Shimoi T, Hamada A, Yamagishi M, Hirai M, Yoshida M, Nishikawa T, Sudo K, Shimomura A, Noguchi E, Yunokawa M, Yonemori K, Shimizu C, Kinoshita T, Fukuda T, Fujiwara Y, Tamura K. PIK3CA mutation profiling in patients with breast cancer, using a highly sensitive detection system. Cancer Sci, 109:2558-2566, 2018
21.Iizumi S, Shimomura A, Shimoi T, Sudo K, Noguchi E, Yonemori K, Shimizu C, Fujiwara Y, Tamura K. Efficacy of capecitabine in patients with locally advanced or metastatic breast cancer with or without prior treatment with fluoropyrimidine: a retrospective study. Cancer Chemother Pharmacol, 82:275-283, 2018
22.Cortes J, Tamura K, DeAngelo DJ, de Bono J, Lorente D, Minden M, Uy GL, Kantarjian H, Chen LS, Gandhi V, Godin R, Keating K, McEachern K, Vishwanathan K, Pease JE, Dean E. Phase I studies of AZD1208, a proviral integration Moloney virus kinase inhibitor in solid and haematological cancers. Br J Cancer, 118:1425-1433, 2018
23.Takano T, Tsurutani J, Takahashi M, Yamanaka T, Sakai K, Ito Y, Fukuoka J, Kimura H, Kawabata H, Tamura K, Matsumoto K, Aogi K, Sato K, Nishio K, Nakagawa K, Saeki T. A randomized phase II trial of trastuzumab plus capecitabine versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and taxanes: WJOG6110B/ELTOP. Breast, 40:67-75, 2018
24.Tamura K, Kodaira M, Shimizu C, Yonemori K, Yunokawa M, Shimomura A, Kobayashi T, Nakano K, Tomomatsu J, Ito Y, Tanaka J, Kuriki H, Gu Z, Takahashi S. Phase I study of taselisib in Japanese patients with advanced solid tumors or hormone receptor-positive advanced breast cancer. Cancer Sci, 109:1592-1601, 2018
25.Kodaira M, Yonemori K, Shimoi T, Yoshida A, Yoshida M, Kitano A, Shimomura A, Yunokawa M, Shimizu C, Takiguchi Y, Fujiwara Y, Tamura K. Prognostic impact of presumed breast or ovarian cancer among patients with unfavorable-subset cancer of unknown primary site. BMC Cancer, 18:176, 2018
26.Bun S, Yunokawa M, Tamaki Y, Shimomura A, Shimoi T, Kodaira M, Shimizu C, Yonemori K, Fujiwara Y, Makino Y, Terakado H, Tamura K. Symptom management: the utility of regional cooling for hand-foot syndrome induced by pegylated liposomal doxorubicin in ovarian cancer. Support Care Cancer, 26:2161-2166, 2018
27.Gambacorti-Passerini C, Orlov S, Zhang L, Braiteh F, Huang H, Esaki T, Horibe K, Ahn JS, Beck JT, Edenfield WJ, Shi Y, Taylor M, Tamura K, Van Tine BA, Wu SJ, Paolini J, Selaru P, Kim TM. Long-term effects of crizotinib in ALK-positive tumors (excluding NSCLC): A phase 1b open-label study. Am J Hematol, 93:607- 614, 2018
28.Udagawa C, Nakamura H, Ohnishi H, Tamura K, Shimoi T, Yoshida M, Yoshida T, Totoki Y, Shibata T, Zembutsu H. Whole exome sequencing to identify genetic markers for trastuzumab-induced cardiotoxicity. Cancer Sci, 109:446-452, 2018
29.Shimoi T, Yoshida M, Kitamura Y, Yoshino T, Kawachi A, Shimomura A, Noguchi E, Yunokawa M, Yonemori K, Shimizu C, Kinoshita T, Ichimura K, Fukuda T, Fujiwara Y, Tamura K. TERT promoter hotspot mutations in breast cancer. Breast Cancer, 25:292-296, 2018
30.Nagumo Y, Maejima A, Toyoshima Y, Komiyama M, Yonemori K, Yoshida A, Fujimoto H. Neoadjuvant crizotinib in ALK-rearranged inflammatory myofibroblastic tumor of the urinary bladder: A case report. Int J Surg Case Rep, 48:1-4, 2018
31.Inagaki C, Shimoi T, Okuma H, Kitano A, Shimomura A, Noguchi E, Kodaira M, Yunokawa M, Yonemori K, Shimizu C, Yoshida A, Fujiwara Y, Tamura K. A case of heavily pretreated metastatic cardiac angiosarcoma treated successfully using eribulin. Anticancer Drugs, 29:97-101, 2018
32.Hirakawa A, Yonemori K, Kinoshita F, Kobayashi Y, Okuma HS, Kawachi A, Tamura K, Fujiwara Y, Rubinstein L, Harris PJ, Takebe N. Potential utility of a longitudinal relative dose intensity of molecularly targeted agents in phase 1 dose-finding trials. Cancer Sci, 109:207-214, 2018
33.Niikura N, Shimomura A, Fukatsu Y, Sawaki M, Ogiya R, Yasojima H, Fujisawa T, Yamamoto M, Tsuneizumi M, Kitani A, Watanabe J, Matsui A, Takahashi Y, Takashima S, Shien T, Tamura K, Saji S, Masuda N, Tokuda Y, Iwata H. Durable complete response in HER2-positive breast cancer: a multicenter retrospective analysis. Breast Cancer Res Treat, 167:81-87, 2018
34.Bun S, Yonemori K, Akagi T, Noguchi E, Shimoi T, Shimomura A, Yunokawa M, Shimizu C, Fujiwara Y, Makino Y, Hayashi Y, Tamura K. Feasibility of olanzapine, multi acting receptor targeted antipsychotic agent, for the prevention of emesis caused by continuous cisplatin- or ifosfamide-based chemotherapy. Invest New Drugs, 36:151-155, 2018
35.Hirakawa A, Nishikawa T, Yonemori K, Shibata T, Nakamura K, Ando M, Ueda T, Ozaki T, Tamura K, Kawai A, Fujiwara Y. Utility of Bayesian Single-Arm Design in New Drug Application for Rare Cancers in Japan: A Case Study of Phase 2 Trial for Sarcoma. Ther Innov Regul Sci, 52:334-338, 2018
36.Nishimura Y, Yoshida A, Yonemori K, Motoi N, Tamura K, Hiraoka N, Mori T. SMARCB-1 deficient squamous cell carcinoma of a mediastinal cyst. Pathol Int, 68:563-566, 2018
37.Elimova E, Wang X, Qiao W, Sudo K, Wadhwa R, Shiozaki H, Shimodaira Y, Planjery V, Charalampakis N, Lee JH, Weston BR, Bhutani MS, Komaki R, Rice DC, Swisher SG, Blum MA, Rogers JE, Skinner HD, Maru DM, Hofstetter WL, Ajani JA. Actionable Locoregional Relapses after Therapy of Localized Esophageal Cancer: Insights from a Large Cohort. Oncology, 94:345-353, 2018
38.Nishimura M, Hayashi M, Mizutani Y, Takenaka K, Imamura Y, Chayahara N, Toyoda M, Kiyota N, Mukohara T, Aikawa H, Fujiwara Y, Hamada A, Minami H. Distribution of erlotinib in rash and normal skin in cancer patients receiving erlotinib visualized by matrix assisted laser desorption/ionization mass spectrometry imaging. Oncotarget, 9:18540-18547, 2018
39.Tanioka M, Fan C, Parker JS, Hoadley KA, Hu Z, Li Y, Hyslop TM, Pitcher BN, Soloway MG, Spears PA, Henry LN, Tolaney S, Dang CT, Krop IE, Harris LN, Berry DA, Mardis ER, Winer EP, Hudis CA, Carey LA, Perou CM. Integrated Analysis of RNA and DNA from the Phase III Trial CALGB 40601 Identifies Predictors of Response to Trastuzumab-Based Neoadjuvant Chemotherapy in HER2-Positive Breast Cancer. Clin Cancer Res, 24:5292- 5304, 2018
40.Tanioka M, Mott KR, Hollern DP, Fan C, Darr DB, Perou CM. Identification of Jun loss promotes resistance to histone deacetylase inhibitor entinostat through Myc signaling in luminal breast cancer. Genome Med, 10:86, 2018
41.Onishi H, Ishida M, Uchida N, Shintani D, Nishikawa T, Hasegawa K, Fujiwara K, Akechi T. Subclinical thiamine deficiency identified by preoperative evaluation in an ovarian cancer patient: Diagnosis and the need for preoperative thiamine measurement. Palliat Support Care, 1-2, 2018
42.Yano M, Asami Y, Nishikawa T, Yoshida S, Kamada K, Katoh T, Teramoto Y, Nakamura Y, Yasuda M. Immune checkpoint inhibitors of CTLA4 and PD-1 for malignant melanoma arising in ovarian cystic teratoma: A case report. Medicine (Baltimore), 97:e12937, 2018
43.Kobayashi K, Matsumoto F, Miyakita Y, Mori T, Shimoi T, Murakami N, Yoshida A, Arakawa A, Omura G, Fukasawa M, Matsumoto Y, Matsumura S, Itami J, Narita Y, Yoshimoto S. Impact of Surgical Margin in Skull Base Surgery for Head and Neck Sarcomas. J Neurol Surg B Skull Base, 79:437-444, 2018
44.Ebata T, Shimoi T, Bun S, Miyake M, Yoshida A, Shimomura A, Noguchi E, Yonemori K, Shimizu C, Fujiwara Y, Narita Y, Tamura K. Efficacy and Safety of Pazopanib for Recurrent or Metastatic Solitary Fibrous Tumor. Oncology, 94:340-344, 2018
