Annual Report 2018
Department of Thoracic Oncology
Yuichiro Ohe, Noboru Yamamoto, Yutaka Fujiwara, Hidehito Horinouchi, Shintaro Kanda, Yasushi Goto, Shuji Murakami, Tatsuya Yoshida, Yuji Matsumoto, Jun Sato, Ryo Morita
Introduction
Lung cancer is the leading cause of cancer death in Japan and worldwide. The incidence of lung cancer in Japan is still increasing, especially in elderly people. The Department of Thoracic Oncology provides care for patients with primary lung cancer, mediastinal tumors, and pleural tumors. The goals of the department are to provide the highest quality treatment and establish new effective treatments against lung cancer and other thoracic malignancies through innovative clinical and translational research. To provide assistance to our patients through multidisciplinary care, the staff members of the department work closely with thoracic surgeons, radiation oncologists, pharmacists, clinical research coordinators, and psychiatrists who have expertise in these areas. The department includes 8 staff physicians. Moreover, residents and trainees from other institutions have joined the Thoracic Oncology Program.
The Team and What We Do
The staff physicians attend outpatient services for thoracic diseases, and the department has approximately 60 beds in the hospital. Inpatient care is carried out by five teams. Each team consists of one staff physician and one or two residents and/or trainee doctors. Protocol and case conferences are scheduled every Monday morning and afternoon, respectively. The journal club is scheduled on Thursday mornings.
Table 1. Number of new patients 2018
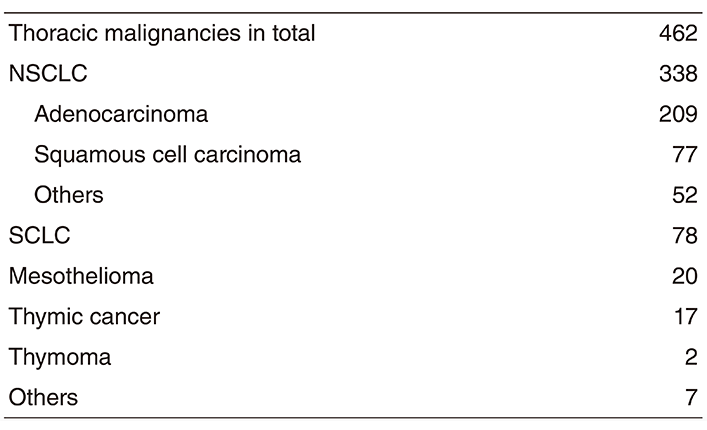

Table 2. Type of procedure in 2018


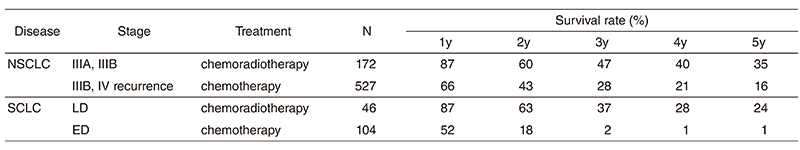
A total of 462 new patients started treatment in 2017, and the backgrounds and initial treatments of these patients are shown in Tables 1 and 2. The initial treatments were chemotherapy in 272 cases, adjuvant chemotherapy after surgery in 43, chemoradiotherapy in 73, curative radiotherapy in four, and supportive care including palliative radiotherapy in 11. Survival of lung cancer patients treated in 2008-2012 in our department is shown in Table 3.
Table 3. Survival of lung cancer patients treated in 2009-2013

Research activities
Research activities of the department can be classified into four categories: (1) multiinstitutional phase III studies to establish new standard treatments against lung cancer; (2) phase I and phase II studies to evaluate new anticancer drugs, (3) pharmacokinetic and pharmacodynamic (PK/PD) studies to investigate interpatient variability, optimal administration schedules and drug-drug interactions; and (4) translational research using clinical samples from bench to bed-side or from bed-side to bench for the development of innovative treatment strategies.
Clinical trials
The department is currently conducting and participating in multi-institutional phase III studies to establish new standard treatments against lung cancer such as the Japan Clinical Oncology Group (JCOG) trials and global trials conducted by pharmaceutical companies. Three JCOG phase III studies, JCOG1201 for elderly EDSCLC, JCOG1206 for high grade neuroendocrine carcinoma and JCOG1404 (AGAIN), a phase III study for EGFR mutation positive NSCLC are ongoing. The department is also participating in a nationwide screening project of lung cancer with rare driver mutation (LC-SCRUM). The department carried out many clinical trials using 3rd generation EGFR-TKIs, anti-PD-1Ab and antiPD-L1Ab.
Education
In 2018, two chief residents and 17 residents joined the department. A monthly research conference is held to discuss clinical and translational research conducted by young doctors.
Future prospects
Recent progression of lung cancer treatment is very rapid. Driver gene alteration targeted therapy such as EGFR-TKIs for EGFR mutation positive lung cancer, ALK inhibitors for ALK fusion gene positive lung cancer, ROS inhibitor for ROS1 fusion gene positive lung cancer and BRAF plus MEK inhibitor for BRAF V600 positive lung cancer are already established as a standard treatment. Other rare driver gene alterations such as RET fusion, MET mutation and NTRAK fusion will be able to identify good targets for treatment of lung cancer. Immunotherapy using anti-PD1Ab and anti-PD-L1 Ab has been established as a standard 2nd or 3rd line treatment for NSCLC. Anti-PD-1 Ab, pembrolizumab and anti-PD-L1 Ab, atezolizumab have been established as a standard 1st line treatment for PD-L1 high expression NSCLC and stage III NSCLC after chemoradiotherapy, respectively. A combination of chemotherapy with an immune check point inhibitor and a combination of an immune check point inhibitor with another immune check point inhibitor will be a standard treatment for lung cancer in the near future. An immune check point inhibitor will also be an incorporated treatment for early stage lung cancer.
List of papers published in 2018
Journal
1. Yoh K, Takamochi K, Shukuya T, Hishida T, Tsuboi M, Sakurai H, Goto Y, Yoshida K, Ohde Y, Okumura S, Ohashi Y, Kunitoh H. Pattern of care in adjuvant therapy for resected Stage I non-small cell lung cancer: real-world data from Japan. Jpn J Clin Oncol, 49:63-68, 2019
2. Kikuchi K, Nozawa K, Yamazaki N, Nakai Y, Higashiyama A, Asano M, Fujiwara Y, Kanda S, Ohe Y, Takashima A, Boku N, Inoue A, Takahashi M, Mori T, Taguchi O, Inoue Y, Mizutani H. Instrumental evaluation sensitively detects subclinical skin changes by the epidermal growth factor receptor inhibitors and risk factors for severe acneiform eruption. J Dermatol, 46:18-25, 2019
3. Mizugaki H, Hamada A, Shibata T, Hosoda F, Nakamura H, Okuma Y, Shukuya T, Umemura S, Horiike A, Fukui T, Kogure Y, Daga H, Urata Y, Yamada K, Saeki S, Fujisaka Y, Nakamura Y, Sato M, Yoshida T, Hotta T, Oizumi S, Fujiwara Y, Ohe Y, Fujiwara Y. Exploration of germline variants responsible for adverse events of crizotinib in anaplastic lymphoma kinase-positive non-small cell lung cancer by target-gene panel sequencing. Lung Cancer, 128:20-25, 2019
4. Watanabe S, Yoshioka H, Sakai H, Hotta K, Takenoyama M, Yamada K, Sugawara S, Takiguchi Y, Hosomi Y, Tomii K, Niho S, Yamamoto N, Nishio M, Ohe Y, Kato T, Takahashi T, Kamada A, Suzukawa K, Omori Y, Enatsu S, Nakagawa K, Tamura T. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line treatment for stage IV squamous nonsmall cell lung cancer: A phase 1b and randomized, open-label, multicenter, phase 2 trial in Japan. Lung Cancer, 129:55-62, 2019
5. Imafuku S, Matsuki T, Mizukami A, Goto Y, de Souza S, Jegou C, Bianco V, Rosillon D, Ito C, Curran D, Holl K. Burden of Herpes Zoster in the Japanese Population with Immunocompromised/ Chronic Disease Conditions: Results from a Cohort Study Claims Database from 2005-2014. Dermatol Ther (Heidelb), 9:117-133, 2019
6. Saito M, Kage H, Ando T, Sawada R, Amano Y, Goto Y, Shinoda Y, Nagase T. Prevalence of bone pain decreases as lymph node stage increases in nonsmall cell lung cancer patients. Curr Probl Cancer, 43:86-91, 2019
7. Ohe Y, Imamura F, Nogami N, Okamoto I, Kurata T, Kato T, Sugawara S, Ramalingam SS, Uchida H, Hodge R, Vowler SL, Walding A, Nakagawa K. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol, 49:29-36, 2019
8. Cho BC, Chewaskulyong B, Lee KH, Dechaphunkul A, Sriuranpong V, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cheng Y, Cho EK, Voon PJ, Lee JS, Mann H, Saggese M, Reungwetwattana T, Ramalingam SS, Ohe Y. Osimertinib versus Standard of Care EGFR TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC: FLAURA Asian Subset. J Thorac Oncol, 14:99-106, 2019
9. Nokihara H, Nishio M, Yamamoto N, Fujiwara Y, Horinouchi H, Kanda S, Horiike A, Ohyanagi F, Yanagitani N, Nguyen L, Yaron Y, Borgman A, Tamura T. Phase 1 Study of Cabozantinib in Japanese Patients With Expansion Cohorts in Non-Small-Cell Lung Cancer. Clin Lung Cancer, 20:e317-e328, 2019
10. Shimomura A, Yamamoto N, Kondo S, Fujiwara Y, Suzuki S, Yanagitani N, Horiike A, Kitazono S, Ohyanagi F, Doi T, Kuboki Y, Kawazoe A, Shitara K, Ohno I, Banerji U, Sundar R, Ohkubo S, Calleja EM, Nishio M. First-in-Human Phase I Study of an Oral HSP90 Inhibitor, TAS-116, in Patients with Advanced Solid Tumors. Mol Cancer Ther, 18:531-540, 2019
11. Itahashi K, Shimizu T, Koyama T, Kondo S, Fujiwara Y, Yamamoto N. Global trends in the distribution of cancer types among patients in oncology phase I trials, 1991-2015. Invest New Drugs, 37:166-174, 2019
12. Yanai T, Iwasa S, Hashimoto H, Ohyanagi F, Takiguchi T, Takeda K, Nakao M, Sakai H, Nakayama T, Minato K, Arai T, Suzuki K, Shimada Y, Nagashima K, Terakado H, Yamamoto N. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol, 23:382-388, 2018
13. Matsubara N, Naito Y, Nakano K, Fujiwara Y, Ikezawa H, Yusa W, Namiki M, Okude T, Takahashi S. Lenvatinib in combination with everolimus in patients with advanced or metastatic renal cell carcinoma: A phase 1 study. Int J Urol, 25:922-928, 2018
14. Hosomi Y, Tanai C, Yoh K, Goto Y, Sakai H, Kato T, Kaburagi T, Nishio M, Kim YH, Inoue A, Hasegawa Y, Isobe H, Tomizawa Y, Mori Y, Minato K, Yamada K, Ohashi Y, Kunitoh H. Characteristics and outcomes of patients with EGFR-mutation positive nonsmall-cell lung cancer receiving gefitinib beyond radiological progression. Expert Opin Pharmacother, 19:1049-1056, 2018
15. Iwasa S, Yamamoto N, Shitara K, Tamura K, Matsubara N, Tajimi M, Lin AB, Asou H, Cai Z, Inoue K, Shibasaki Y, Saito K, Takai H, Doi T. Dose-finding study of the checkpoint kinase 1 inhibitor, prexasertib, in Japanese patients with advanced solid tumors. Cancer Sci, 109:3216-3223, 2018
16. Gridelli C, de Castro Carpeno J, Dingemans AC, Griesinger F, Grossi F, Langer C, Ohe Y, Syrigos K, Thatcher N, Das-Gupta A, Truman M, Donica M, Smoljanovic V, Bennouna J. Safety and Efficacy of Bevacizumab Plus Standard-of-Care Treatment Beyond Disease Progression in Patients With Advanced Non-Small Cell Lung Cancer: The AvaALL Randomized Clinical Trial. JAMA Oncol, 4:e183486, 2018
17. Kimbara S, Fujiwara Y, Iwama S, Ohashi K, Kuchiba A, Arima H, Yamazaki N, Kitano S, Yamamoto N, Ohe Y. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci, 109:3583-3590, 2018
18. Mizutani T, Ando M, Mizusawa J, Nakamura K, Fukuda H, Tsukada H, Abe T, Takeda K, Yokoyama A, Nakamura S, Nakagawa K, Yamamoto N, Ohe Y. Prognostic value of Lung Cancer Subscale in older patients with advanced non-small cell lung cancer: An integrated analysis of JCOG0207 and JCOG0803/WJOG4307L (JCOG1414A). J Geriatr Oncol, 9:583-588, 2018
19. Shinno Y, Goto Y, Watanabe S, Sato J, Morita R, Matsumoto Y, Murakami S, Kanda S, Horinouchi H, Fujiwara Y, Yamamoto N, Ohe Y. Evaluation of time to failure of strategy as an alternative surrogate endpoint in patients with lung cancer with EGFR mutations. ESMO Open, 3:e000399, 2018
20. Hashimoto H, Abe M, Yanai T, Yamaguchi T, Zenda S, Uchitomi Y, Fukuda H, Mori M, Iwasa S, Yamamoto N, Ohe Y. Study protocol for J-SUPPORT 1604 (J-FORCE): a randomized, double blind, placebo-controlled Phase III study evaluating olanzapine (5 mg) plus standard triple antiemetic therapy for prevention of chemotherapy induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy. Jpn J Clin Oncol, 48:950-952, 2018
21. Sekine K, Kanda S, Goto Y, Horinouchi H, Fujiwara Y, Yamamoto N, Motoi N, Ohe Y. Change in the lymphocyte-to-monocyte ratio is an early surrogate marker of the efficacy of nivolumab monotherapy in advanced non-small-cell lung cancer. Lung Cancer, 124:179-188, 2018
22. Yamamoto N, Kenmotsu H, Goto K, Takeda K, Kato T, Takeda M, Horinouchi H, Saito I, Sarashina A, Tanaka T, Morsli N, Nakagawa K. An open-label feasibility study of nintedanib combined with docetaxel in Japanese patients with locally advanced or metastatic lung adenocarcinoma after failure of first-line chemotherapy. Cancer Chemother Pharmacol, 82:685-694, 2018
23. Atagi S, Mizusawa J, Ishikura S, Takahashi T, Okamoto H, Tanaka H, Goto K, Nakagawa K, Harada M, Takeda Y, Nogami N, Fujita Y, Kasai T, Kishi K, Sawa T, Takeda K, Tomii K, Satouchi M, Seto T, Ohe Y. Chemoradiotherapy in Elderly Patients With NonSmall-Cell Lung Cancer: Long-Term Follow-Up of a Randomized Trial (JCOG0301). Clin Lung Cancer, 19:e619-e627, 2018
24. Goto Y. Tumor Mutation Burden: Is It Ready for the Clinic? J Clin Oncol, JCO2018793398, 2018
25. Hida T, Seto T, Horinouchi H, Maemondo M, Takeda M, Hotta K, Hirai F, Kim YH, Matsumoto S, Ito M, Ayukawa K, Tokushige K, Yonemura M, Mitsudomi T, Nishio M. Phase II study of ceritinib in alectinib-pretreated patients with anaplastic lymphoma kinase-rearranged metastatic non-small-cell lung cancer in Japan: ASCEND-9. Cancer Sci, 109:2863-2872, 2018
26. Watanabe K, Yasumoto A, Amano Y, Kage H, Goto Y, Yatomi Y, Takai D, Nagase T. Mean platelet volume and lymphocyte-to-monocyte ratio are associated with shorter progression-free survival in EGFR-mutant lung adenocarcinoma treated by EGFR tyrosine kinase inhibitor. PLoS ONE, 13:e0203625, 2018
27. Ohe Y. Patient-reported outcomes in a phase II study of alectinib. ESMO Open, 3:e000412, 2018
28. Tanaka M, Horinouchi H, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Ohe Y. Reduction in nephrotoxicities using short hydration for chemotherapy containing cisplatin: a consecutive analysis of 467 patients with thoracic malignancies. ESMO Open, 3:e000342, 2018
29. Fujiwara Y, Takeda M, Yamamoto N, Nakagawa K, Nosaki K, Toyozawa R, Abe C, Shiga R, Nakamaru K, Seto T. Safety and pharmacokinetics of DS-6051b in Japanese patients with nonsmall cell lung cancer harboring ROS1 fusions: a phase I study. Oncotarget, 9:23729-23737, 2018
30. Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto T, Yang JJ, Yamamoto N, Ahn MJ, Takahashi T, Yamanaka T, Kemner A, Roychowdhury D, Paolini J, Usari T, Wilner KD, Goto K. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol, 36:1405-1411, 2018
31. Yoshida K, Fujiwara Y, Goto Y, Kohno T, Yoshida A, Tsuta K, Ohe Y. The first case of SMARCB1 (INI1) - deficient squamous cell carcinoma of the pleura: a case report. BMC Cancer, 18:398, 2018
32. Ogawa A, Kondo K, Takei H, Fujisawa D, Ohe Y, Akechi T. Decision-Making Capacity for Chemotherapy and Associated Factors in Newly Diagnosed Patients with Lung Cancer. Oncologist, 23:489-495, 2018
33. Horinouch H. Precision radiotherapy for patients with locally advanced non-small cell lung cancer in the era of immunotherapy and precision medicine. Transl Lung Cancer Res, 7:S146-S148, 2018
34. Watanabe J, Furuya N, Fujiwara Y. Appearance of a BRAF Mutation Conferring Resistance to Crizotinib in Non-Small Cell Lung Cancer Harboring Oncogenic ROS1 Fusion. J Thorac Oncol, 13:e66-e69, 2018
35. Inoki K, Nakajima T, Nonaka S, Abe S, Suzuki H, Yoshinaga S, Oda I, Yamada M, Takatsu M, Yoshida H, Taniguchi H, Sekine S, Ohe Y, Saito Y. Feasibility of endoscopic resection using bipolar snare for nonampullary duodenal tumours in familial adenomatous polyposis patients. Fam Cancer, 17:517-524, 2018
36. Yamazaki N, Tsutsumida A, Takahashi A, Namikawa K, Yoshikawa S, Fujiwara Y, Kondo S, Mukaiyama A, Zhang F, Kiyohara Y. Phase 1/2 study assessing the safety and efficacy of dabrafenib and trametinib combination therapy in Japanese patients with BRAF V600 mutation-positive advanced cutaneous melanoma. J Dermatol, 45:397-407, 2018
37. Fujiwara Y, Yamazaki N, Kiyohara Y, Yoshikawa S, Yamamoto N, Tsutsumida A, Nokihara H, Namikawa K, Mukaiyama A, Zhang F, Tamura T. Safety, tolerability, and pharmacokinetic profile of dabrafenib in Japanese patients with BRAF (V600) mutation-positive solid tumors: a phase 1 study. Invest New Drugs, 36:259-268, 2018
38. Itahashi K, Kondo S, Kubo T, Fujiwara Y, Kato M, Ichikawa H, Koyama T, Tokumasu R, Xu J, Huettner CS, Michelini VV, Parida L, Kohno T, Yamamoto N. Evaluating Clinical Genome Sequence Analysis by Watson for Genomics. Front Med, 5:305, 2018
39. Kato M, Nakamura H, Nagai M, Kubo T, Elzawahry A, Totoki Y, Tanabe Y, Furukawa E, Miyamoto J, Sakamoto H, Matsumoto S, Sunami K, Arai Y, Suzuki Y, Yoshida T, Tsuchihara K, Tamura K, Yamamoto N, Ichikawa H, Kohno T, Shibata T. A computational tool to detect DNA alterations tailored to formalin-fixed paraffin-embedded samples in cancer clinical sequencing. Genome Med, 10:44, 2018
