Annual Report 2018
Department of Dermatologic Oncology
Naoya Yamazaki, Akira Takahashi, Kenjiro Namikawa, Haruki Mizuta
Introduction
The Department of Dermatologic Oncology has consistently served as the core hospital for the establishment of treatment strategies for malignant skin tumors since the National Cancer Center opened in 1962, and over 4,000 cases of malignant melanoma have been accumulated thus far; an impressive number for a hospital or research institution in Japan. Today, patients are referred from throughout Japan. Of particular note, the number of patients with malignant melanoma was 187, which was approximately twice the number 10 years ago. Most of the patients are examined and treated for skin cancer including malignant melanoma. Surgery is the main treatment modality for skin cancer, and multi-disciplinary treatments, consisting of immunotherapy, targeted therapy, chemotherapy, and radiotherapy, are also routinely carried out. But these days, especially, the importance of immunotherapy is increasing. Melanoma is at the center of development of immune therapy. This Department plays an active role in multicenter trials for new agents for skin cancer all over Japan.
The Team and What We Do
The Department has four staff dermatologic oncologists and five residents. We are also engaged in routine outpatient activities on Wednesdays and Thursdays in the National Cancer Center East.
Our department is a high-volume center, where we have seen an average of 180 patients with malignant melanoma annually in the last 5 years. This is the result of the creation of a national network to develop treatment for malignant skin tumors, and nivolumab, an antiPD-1 antibody, was approved as a therapeutic agent for malignant melanoma in Japan for the first time in the world as a result of vigorous development of new drugs.
At all times, about 20 patients are hospitalized to undergo surgery, chemotherapy, or radiation therapy. In 2018, 339 operations were performed including 150 operations under general anesthesia.
For first-line systemic therapy of unresectable or metastatic melanoma, recommended treatment options include checkpoint immunotherapy, BRAF-targeted therapy for patients with BRAFmutated disease, or clinical trials.
Checkpoint immunotherapy options in this setting include anti-PD-1 monotherapy or a combination of ipilimumab and nivolumab. Checkpoint inhibitors have been shown to be effective regardless of BRAF-mutation status.
For almost all patients with BRAF-mutant metastatic disease, BRAF-targeted therapy first-line options include BRAF/MEK inhibitor combination therapy with dabrafenib/trametinib.
Rounds are made and case presentations are held every morning. A weekly dermatologic oncology conference is held every Monday to discuss the therapeutic principles for outpatients and inpatients. A clinicopathological conference that focuses on surgically removed skin specimens is held with pathologists once a month.
In addition, we have treated advanced cases of mucosal melanoma patients in the nasal cavity, genital lesions, perianal lesions, and uveal melanoma even if our origins are "dermatologic".
Research activities
Malignant skin tumors are mainly treated by surgery (appended table). However, in recent years, several new drugs have been developed rapidly overseas for the treatment of malignant melanoma, and our department is conducting many clinical studies and trials; the major ones are described below.
- A study of the prognosis of malignant melanoma and the relevant gene expressions in the tumor
- Development of the Hyperspectral Imager
- A single-arm confirmatory trial of pazopanib in patients with paclitaxel-pretreated primary cutaneous angiosarcoma (JCOG1605)
- Real-world efficacy of anti-PD-1 antibodies in advanced acral melanoma patients: a retrospective, multicenter study (JAMP study)
- A phase I clinical trial of intratumoral administration of GEN0101 in patients with advanced malignant melanoma.
- A phase II study of combined HF10 plus ipilimumab therapy in patients with advanced skin melanoma.
- A multinational Phase III clinical trial of nivolumab in combination with anti-LAG-3 antibody for advanced malignant melanoma
- A randomized phase III trial of adjuvant therapy with locoregional IFN-beta versus observation in stage II/III cutaneous melanoma (JCOG1309)
- Confirmatory trial of non-amputative digit preservation surgery in subungual melanoma (JCOG1602)
The efficacy and safety of combined nivolumab (an anti-PD-1 antibody) plus ipilimumab (an anti-CTLA-4 antibody) therapy in Japanese patients with malignant melanoma were clarified.
The efficacy and safety of encorafenib (a BRAF inhibitor) and binimetinib (a MEK inhibitor) for malignant melanoma were clarified.
We reported that the incidence of severe skin disorders increased markedly when vemurafenib (a BRAF inhibitor) was administered after administration of nivolumab.
Adjuvant therapy of melanoma for Japanese patients was established.
Based on the results of a retrospective study of primary cutaneous angiosarcoma of the head and neck, we reported that the most effective treatment was surgery plus postoperative radiotherapy.
Clinical trials
Table 3 shows our clinical trials
Education
Currently, training in routine clinical practice under skilled guidance is ongoing for four resident physicians. In addition, conferences with the departments of oncology, radiotherapy and pathology are held on a regular basis. The resident physicians and the oncology trainee made a total of 15 presentations in domestic academic conferences, two presentations in international academic conferences and also published four papers, two of them were international academic papers in English.
Future prospects
With the continuing rapid progress in drug therapy for advanced cutaneous malignant melanoma, we are now facing an era where the indications of drug therapy for malignant melanoma are being expanded to include combination therapies and adjuvant therapies. The currently adopted treatments for malignant melanoma and Merkel cell carcinoma in Japan are the most advanced in the world. On the other hand, information and treatment options are limited for squamous cell carcinoma, cutaneous angiosarcoma, etc. Therefore, there is an urgent demand for the development of new treatments and it is necessary to continue developmental activities in cooperation with major foreign institutions.
Table 1. Number of New Patients
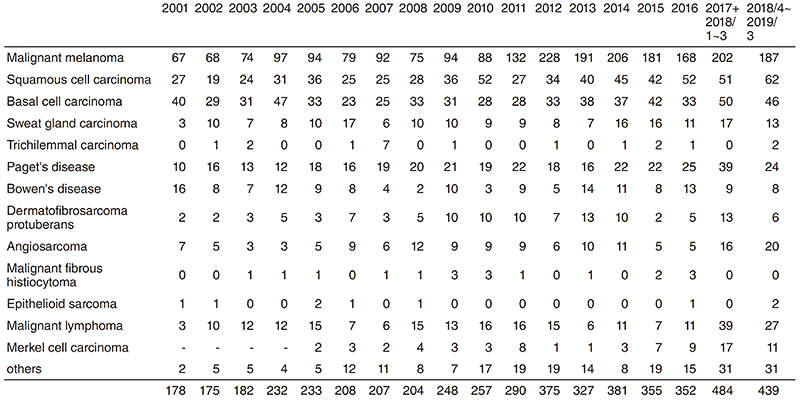

Table 2. Operative Procedures (total number) in 2018/4~2019/3
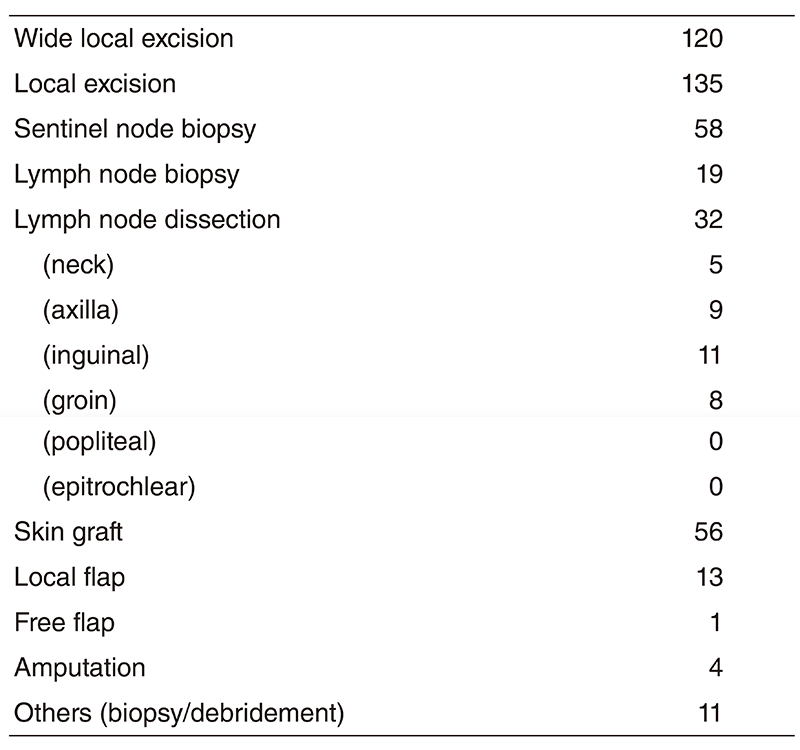

Table 3. New Agent Studies in 2018/4~2019/3

List of papers published in 2018
Journal
1. Kikuchi K, Nozawa K, Yamazaki N, Nakai Y, Higashiyama A, Asano M, Fujiwara Y, Kanda S, Ohe Y, Takashima A, Boku N, Inoue A, Takahashi M, Mori T, Taguchi O, Inoue Y, Mizutani H. Instrumental evaluation sensitively detects subclinical skin changes by the epidermal growth factor receptor inhibitors and risk factors for severe acneiform eruption. J Dermatol, 46:18-25, 2019
2. Ishida H, Shimizu Y, Sakaguchi H, Nitanda H, Kaneko K, Yamazaki N, Yanagihara A, Taguchi R, Sakai F, Yasuda M, Kobayashi K. Distinctive clinicopathological features of adenocarcinoma in situ and minimally invasive adenocarcinoma of the lung: A retrospective study. Lung Cancer, 129:16-21, 2019
3. Tanaka R, Yonemori K, Hirakawa A, Kinoshita F, Kobayashi Y, Yamazaki N, Fujimoto M, Tamura K, Fujiwara Y. Anticancer Agent-Induced Life-Threatening Skin Toxicities: A Database Study of Spontaneous Reporting Data. Oncologist, 24:266-272, 2019
4. Namikawa K, Yamazaki N. Targeted Therapy and Immunotherapy for Melanoma in Japan. Curr Treat Options Oncol, 20:7, 2019
5. Yamazaki N, Oomuku Y, Mishiro I, Soeda J. Pre-emptive skin treatments to prevent skin toxicity caused by anti-EGFR antibody: the real-world evidence in Japan. Future Oncol, 14:3163- 3174, 2018
6. Uhara H, Kiyohara Y, Tsuda A, Takata M, Yamazaki N. Characteristics of adverse drug reactions in a vemurafenib early post-marketing phase vigilance study in Japan. Clin Transl Oncol, 20:169- 175, 2018
7. Yamazaki N, Tsutsumida A, Takahashi A, Namikawa K, Yoshikawa S, Fujiwara Y, Kondo S, Mukaiyama A, Zhang F, Kiyohara Y. Phase 1/2 study assessing the safety and efficacy of dabrafenib and trametinib combination therapy in Japanese patients with BRAF V600 mutation-positive advanced cutaneous melanoma. J Dermatol, 45:397-407, 2018
8. Kiyohara Y, Uhara H, Ito Y, Matsumoto N, Tsuchida T, Yamazaki N. Safety and efficacy of nivolumab in Japanese patients with malignant melanoma: An interim analysis of a postmarketing surveillance. J Dermatol, 45:408-415, 2018
9. Muto Y, Ng W, Namikawa K, Takahashi A, Tsutsumida A, Nishida M, Yamazaki N. Success of rechallenging dabrafenib and trametinib combination therapy after trametinib-induced rhabdomyolysis: a case report. Melanoma Res, 28:151-154, 2018
10. Oashi K, Namikawa K, Tsutsumida A, Takahashi A, Itami J, Igaki H, Inaba K, Yamazaki N. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face -a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol, 44:823- 829, 2018
11. Takahashi A, Tsutsumida A, Namikawa K, Yamazaki N. Fulminant type 1 diabetes associated with nivolumab in a patient with metastatic melanoma. Melanoma Res, 28:159-160, 2018
12. Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, Garbe C, Schadendorf D, Krajsova I, Gutzmer R, Chiarion-Sileni V, Dutriaux C, de Groot JWB, Yamazaki N, Loquai C, Moutouh-de Parseval LA, Pickard MD, Sandor V, Robert C, Flaherty KT. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol, 19:603-615, 2018
13. Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, Garbe C, Schadendorf D, Krajsova I, Gutzmer R, Chiarion Sileni V, Dutriaux C, de Groot JWB, Yamazaki N, Loquai C, Moutouh-de Parseval LA, Pickard MD, Sandor V, Robert C, Flaherty KT. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol, 19:1315-1327, 2018
14. Fujiwara Y, Yamazaki N, Kiyohara Y, Yoshikawa S, Yamamoto N, Tsutsumida A, Nokihara H, Namikawa K, Mukaiyama A, Zhang F, Tamura T. Safety, tolerability, and pharmacokinetic profile of dabrafenib in Japanese patients with BRAF (V600) mutation-positive solid tumors: a phase 1 study. Invest New Drugs, 36:259-268, 2018
15. Namikawa K, Kiyohara Y, Takenouchi T, Uhara H, Uchi H, Yoshikawa S, Takatsuka S, Koga H, Wada N, Minami H, Hatsumichi M, Asada S, Namba Y, Yamazaki N. Efficacy and safety of nivolumab in combination with ipilimumab in Japanese patients with advanced melanoma: An open-label, single-arm, multicentre phase II study. Eur J Cancer, 105:114-126, 2018
