Annual Report 2018
Department of Pediatric Oncology
Chitose Ogawa, Tadashi Kumamoto, Ayumu Arakawa, Masanaka Sugiyama, Tomoko Sonoda, Sae Ishimaru, Miho Nakajima, Nami Shirakawa, Kayoko Tao
Introduction
Pediatric oncology includes a wide variety of malignancies in children and adolescents such as acute leukemia and malignant lymphoma, as well as solid tumors including osteosarcoma, soft tissue sarcoma, neuroblastoma, liver tumor and retinoblastoma. Many diseases are usually chemo-sensitive and curable with appropriate treatment. The common approach to these diseases is a "risk-adapted therapy" strategy considering long-term life expectancy. In the Department of Pediatric Oncology, patients with pediatric malignancies are managed by four pediatric oncologists and a pediatric surgeon. Although pediatric oncologists mainly treat and manage patients, a multidisciplinary team approach including radiation oncologists, orthopedic surgeons, ophthalmologic surgeons and others is incorporated for the treatment. To achieve treatment completion and optimal quality of hospital life for children, pediatric nurse specialists, teachers, childcare staff, psychologists and psychiatrists also join our team. For young patients, educational opportunities ranging from elementary school to high school are available in the pediatric ward, where ten teachers work daily
The Team and What We Do
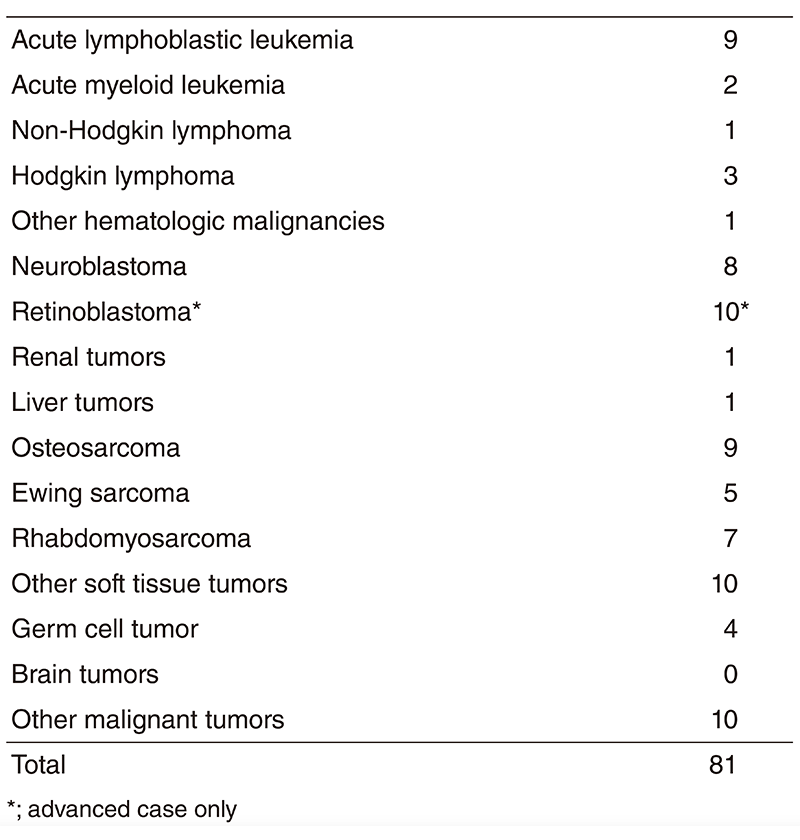
More than 100 new patients come to our department and about 80 patients start the treatment every year. The number of patients is shown in Table 1. Our daily activities in the pediatric outpatient clinic are to manage new patients, to treat patients with chemotherapy or blood transfusion and to provide follow-up care for patients who have completed intensive treatment. Patients receive multidisciplinary therapy, including surgical removal of the tumor, radiation therapy, chemotherapy and sometimes SCT, as indicated.
A Pediatric Conference is held every morning, mainly to decide on individual treatment plans. The pediatric staff and trainees discuss various issues regarding pediatric inpatients on daily rounds. Inter-department conferences in cooperation with orthopedics, radiation oncology, and palliative care are individually scheduled every two weeks.
Table 1. Number of patients in 2018

Research activities
1. For newly diagnosed patients, we participate in several multicenter studies in Japan Children's Cancer Group (JCCG), including those by Japan Ewing Sarcoma Study Group (JESS), Rhabdomyosarcoma Study Group (JRSG) and Japanese Pediatric Leukemia/ Lymphoma Study Group (JPLSG).
2. For relapsed patients, we are actively involved in the development of new drugs and treatments including off-label and unapproved medications.
3. For individualized treatment, we expanded the TOP-GEAR project, implementation of clinical sequencing, to children and started the recruitment of pediatric patients. Eightyseven samples had been analyzed by the end of March 2019 and the results were explained to each patient.
4. For provision of a similar environment during the treatment to that of before patients' disease onset, we plan to construct a medical care system through the use of appropriate medical and social resources in their local communities.
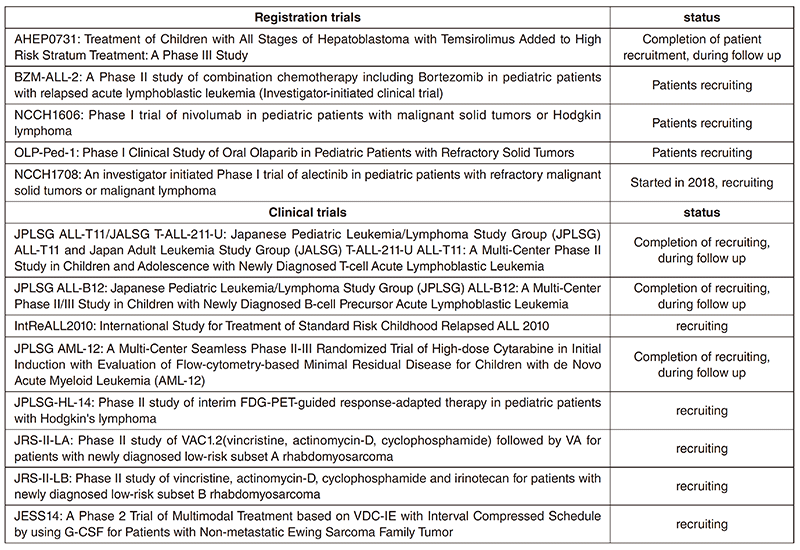
Clinical trials
In 2018, we were doing 13 clinical trials, including early phase trials, an international study and cooperative studies. All clinical trials are listed in Table 2. The five trials, including one started in 2018, are investigator-initiated registration-directed clinical trials conducted under the Pharmaceutical Affairs Law in Japan. Two international cooperative trials are IntReALL2010, which is in collaboration with International BFM group in Europe, and is ongoing, and AHEP 0731 with Children's Oncology Group in the USA, completed patients recruitment.
Education
We provide personnel training and education for the skills of diagnosis and management for pediatric hematological malignancies and solid tumors. Residents also learn skills to treat not only newly diagnosed patients but also relapsed or refractory patients with global standard therapy. In addition, senior residents acquire abilities to plan studies for new agents or new therapies, which we regard as an important role of this center.
Future prospects
We promote development of therapies for pediatric malignancies as a top priority. For this mission, we need to plan clinical or registration trials in cooperation with domestic and international centers as a core institution in Japan.
Our other mission is to provide individualized medicine for children with cancer. For this aim, we plan to expand the subjects in the comprehensive genetic testing project in our center to the pediatric age group. In addition, we promote clinical trials using molecular targeted agents for pediatric malignancies.
List of papers published in 2018
Journal
1. Sunami K, Ichikawa H, Kubo T, Kato M, Fujiwara Y, Shimomura A, Koyama T, Kakishima H, Kitami M, Matsushita H, Furukawa E, Narushima D, Nagai M, Taniguchi H, Motoi N, Sekine S, Maeshima A, Mori T, Watanabe R, Yoshida M, Yoshida A, Yoshida H, Satomi K, Sukeda A, Hashimoto T, Shimizu T, Iwasa S, Yonemori K, Kato K, Morizane C, Ogawa C, Tanabe N, Sugano K, Hiraoka N, Tamura K, Yoshida T, Fujiwara Y, Ochiai A, Yamamoto N, Kohno T. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci, 110:1480-1490, 2019
2. Takagi M, Ogawa C, Aoki-Nogami Y, Iehara T, Ishibashi E, Imai M, Kihara T, Nobori K, Hasebe K, Mizutani S, Kimura T, Nagata M, Yasuhara M, Yoshimura K, Yorozu P, Hosoi H, Koike R. Phase I clinical study of oral olaparib in pediatric patients with refractory solid tumors: study protocol. BMC Pediatr, 19:31, 2019
3. Saito Y, Kumamoto T, Arima T, Shirakawa N, Ishimaru S, Sonoda T, Nakajima M, Sugiyama M, Arakawa A, Hashimoto H, Makino Y, Ogawa C, Yamaguchi M. Evaluation of aprepitant and fosaprepitant in pediatric patients. Pediatr Int, 61:235-239, 2019
4. Handa A, Nozaki T, Makidono A, Okabe T, Morita Y, Fujita K, Matsusako M, Kono T, Kurihara Y, Hasegawa D, Kumamoto T, Ogawa C, Yuza Y, Manabe A. Pediatric oncologic emergencies: Clinical and imaging review for pediatricians. Pediatr Int, 61:122- 139, 2019
5. Kumamoto T, Aoki Y, Sonoda T, Yamanishi M, Arakawa A, Sugiyama M, Shirakawa N, Ishimaru S, Saito Y, Maeshima A, Maeda M, Ogawa C. A case of recurrent histiocytic sarcoma with MAP2K1 pathogenic variant treated with the MEK inhibitor trametinib. Int J Hematol, 109:228-232, 2019
6. Yoshida S, Ogawa C, Shimizu K, Kobayashi M, Inoguchi H, Oshima Y, Dotani C, Nakahara R, Kato M. Japanese physicians' attitudes toward end-of-life discussion with pediatric patients with cancer. Support Care Cancer, 26:3861-3871, 2018
7. Saito Y, Kumamoto T, Shiraiwa M, Sonoda T, Arakawa A, Hashimoto H, Tamai I, Ogawa C, Terakado H. Cyclophosphamide-induced hemorrhagic cystitis in young patients with solid tumors: A single institution study. Asia Pac J Clin Oncol, 14:e460-e464, 2018
8. Takahashi H, Kajiwara R, Kato M, Hasegawa D, Tomizawa D, Noguchi Y, Koike K, Toyama D, Yabe H, Kajiwara M, Fujimura J, Sotomatsu M, Ota S, Maeda M, Goto H, Kato Y, Mori T, Inukai T, Shimada H, Fukushima K, Ogawa C, Makimoto A, Fukushima T, Ohki K, Koh K, Kiyokawa N, Manabe A, Ohara A. Treatment outcome of children with acute lymphoblastic leukemia: the Tokyo Children's Cancer Study Group (TCCSG) Study L04-16. Int J Hematol, 108:98-108, 2018
9. Aruga Y, Arakawa A, Ono K, Ogawa C, Matsushita H. Pseudo-Chediak-Higashi granules and Auer rods in mixed phenotype acute leukaemia, T/myeloid, not otherwise specified. Br J Haematol, 180:175, 2018
10. Miyahara H, Miyakawa K, Nishida H, Yano S, Sonoda T, Suenobu SI, Izumi T, Daa T, Ihara K. Unique cell tropism of HHV-6B in an infantile autopsy case of primary HHV-6B encephalitis. Neuropathology, 2018
11. Nakagawa R, Hosokawa-Tsuji A, Aoki Y, Takasawa K, Maru M, Nakajima K, Sutani A, Miyakawa Y, Tomizawa D, Kashimada K, Morio T. Total body irradiation for hematopoietic stem cell transplantation during early childhood is associated with the risk for diabetes mellitus. Endocrine, 61:76-82, 2018
12. Noguchi Y, Tomizawa D, Hiroki H, Miyamoto S, Tezuka M, Miyawaki R, Tanaka-Kubota M, Okano T, Kobayashi C, Mitsuiki N, Aoki Y, Imai K, Kajiwara M, Kanegane H, Morio T, Takagi M. Hematopoietic cell transplantation for myeloid/NK cell precursor acute leukemia in second remission. J Clin Case Rep, 6:1023- 1028, 2018
13. Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, Fang J, Rampersaud S, Hoshino A, Matei I, Kenific CM, Nakajima M, Mutvei AP, Sansone P, Buehring W, Wang H, Jimenez JP, Cohen-Gould L, Paknejad N, Brendel M, Manova-Todorova K, Magalhaes A, Ferreira JA, Osorio H, Silva AM, Massey A, Cubillos-Ruiz JR, Galletti G, Giannakakou P, Cuervo AM, Blenis J, Schwartz R, Brady MS, Peinado H, Bromberg J, Matsui H, Reis CA, Lyden D. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol, 20:332-343, 2018
14. Hoseini SS, Guo H, Wu Z, Hatano MN, Cheung NV. A potent tetravalent T-cell-engaging bispecific antibody against CD33 in acute myeloid leukemia. Blood Adv, 2:1250-1258, 2018
15. Kobayashi K, Matsumoto F, Miyakita Y, Mori T, Shimoi T, Murakami N, Yoshida A, Arakawa A, Omura G, Fukasawa M, Matsumoto Y, Matsumura S, Itami J, Narita Y, Yoshimoto S. Impact of Surgical Margin in Skull Base Surgery for Head and Neck Sarcomas. J Neurol Surg B Skull Base, 79:437-444, 2018
