Annual Report 2018
Innovation Center for Supportive, Palliative and Psychosocial Care
Yosuke Uchitomi, Takuhiro Yamaguchi, Akiko Hanai, Maki Minemura, Asami Satake, Miyuki Kanamaru, Yutaka Matsuoka, Sadamoto Zenda, Eriko Satomi, Ken Shimizu, Masashi Kato, Maiko Fujimori, Ayako Sato, Masako Okamura, Miyuki Odawara, Tempei Miyaji
Introduction
We have built J-SUPPORT (Japan Supportive, Palliative and Psychosocial Oncology Group) as an open hub for multi-institutional collaborative clinical research for supportive, palliative and psychosocial care and started research management throughout Japan. (Figure 1).
Figure 1. Organization of J-SUPPORT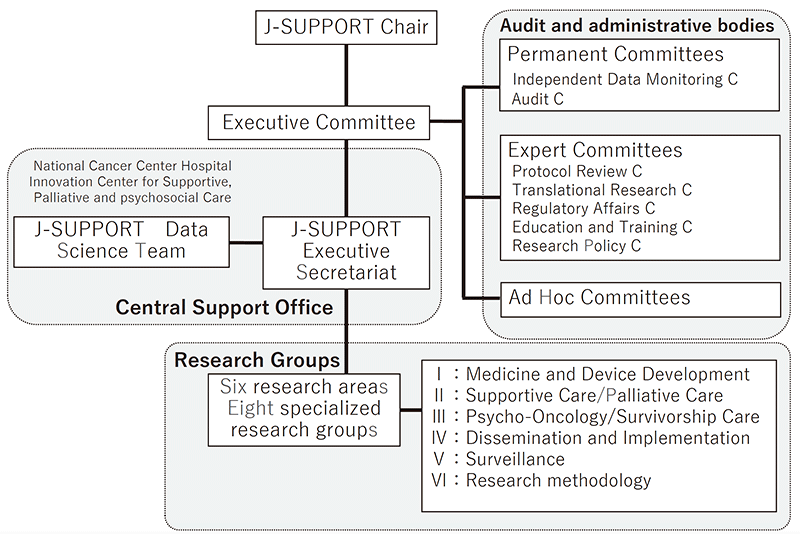

The Team and What We Do
We cooperate in providing consultation services and expert advice on clinical research design and statistical analysis to investigators as they launch new research projects in the field of supportive, palliative and psychosocial care. This service includes face-to-face clinical design and biostatistics consultation, and we adjust collaborative studies with other study groups or institutions. We also conduct a protocol review committee and educational seminar.
Research activities
Seven intervention tests were conducted. Two trials ((1) delirium after surgery: 195 patients and (2) chemotherapy induced nausea and vomiting: 697 patients) completed patient registration. Two new trials ((3) fear of recurrence: 187/444 patients, (4) communication: 50/360 patients) have been started. Five trials of 8/50 patients are under patient registration; (5) radiation dermatitis: 169/210 patients, and (6) early palliative care: 171/206 patients, (7) chemotherapy induced hand foot syndrome. One trial (recommendation for screening of persons with disabilities) is being prepared. A total of 631 patients were registered in FY2018 (816 patients in FY2017). In addition, a supportive care research policy and development strategy matrix were created and published on the website as a research foundation. There were research consultations 29 in FY2018 (7 in FY2017).
Clinical trials
- A Scripted Video-Vignette Study to Explore the Best Practice in Prognostic Disclosure
- Topical Steroid versus placebo for the prevention of radiation dermatitis in head and neck cancer patients receiving chemoradiotherapy: a phase III, randomized, double-blind trial
- Nurse-led, screening-triggered early specialized palliative care intervention program for advanced lung cancer patient randomized controlled trial
- Efficacy and safety of olanzapine 5mg in combination with standard antiemetic therapy for the treatment of chemotherapy - induced nausea and vomiting in patients receiving cisplatin based highly emetogenic chemotherapy: a randomized, double-blind, placebo controlled, phase III clinical trial
- Yokukansan for perioperative psychiatric symptoms in cancer patients undergoing high invasive surgery: a randomized, double-blind, placebo-controlled trial
- Efficacy of prophylactic use of hydrocolloid dressing for hand-foot skin reaction by multi targeted kinase inhibitors: a phase III randomized, self-controlled study
- Smartphone-based problem-solving treatment and behavioral activation intervention to reduce fear of recurrence in breast cancer patients - SMILE study: a randomized controlled trial
- A randomized controlled trial of an integrated support program for promoting empathetic communication among rapid progressive cancer patients, families and physicians
(Cohort study)
- Quality of palliative care for end-of-life cancer patients and retrospective cohort study of unsolved clinical questions of palliative care from administrative claims data
Education
Training for young researchers
- The second J-SUPPORT clinical research concept development workshop was held from January 11 to 12, 2019, and 23 researchers from various professions participated.
J-SUPPORT seminar
- The 3rd "Introduction of data centers managed by researchers and their characteristics" April 13, 2018, 37 participated.
- The 4th "Design and practice of clinical research using PRO" September 13, 2018, 53 participated.
Co-sponsored event
- Dissemination and Implementation Science Society 1st Academic Meeting (November 18, 2018; participants: approx. 200)
- Tokyo University Graduate School of Medicine "Clinical Research Methodology" (September 7, 2018; participants: approx. 200)
- 1st Young Researcher Development Seminar (participants: 7), Psycho-Oncology Training Seminar [Clinical Research Course for PsychoOncologist] (sponsored by the Japan PsychoOncology Society [JPOS]) (September 20, 2018; participants: 7)
Future prospects
We continuously plan research consultation at the immature stage before the protocol concept. We not only conduct clinical research, but also plan to support research activities through a regular protocol review committee and educational training.
List of papers published in 2018
Journal
1. Wada S, Sadahiro R, Matsuoka YJ, Uchitomi Y, Yamaguchi T, Shimizu K. Yokukansan for perioperative psychiatric symptoms in cancer patients undergoing high invasive surgery J-SUPPORT 1605 (ProD Study): study protocol for a randomized controlled trial. Trials, 20:110, 2019
2. Mori M, Fujimori M, Ishiki H, Nishi T, Hamano J, Otani H, Uneno Y, Oba A, Morita T, Uchitomi Y. The Effects of Adding Reassurance Statements: Cancer Patients' Preferences for Phrases in End-ofLife Discussions. J Pain Symptom Manage, 57:1121-1129, 2019
3. Mori M, Fujimori M, Ishiki H, Nishi T, Hamano J, Otani H, Uneno Y, Oba A, Morita T, Uchitomi Y. Adding a Wider Range and "Hope for the Best, and Prepare for the Worst" Statement: Preferences of Patients with Cancer for Prognostic Communication. Oncologist, 24:e943-e952, 2019
4. Shibayama O, Yoshiuchi K, Inagaki M, Matsuoka Y, Yoshikawa E, Sugawara Y, Akechi T, Wada N, Imoto S, Murakami K, Ogawa A, Uchitomi Y. Long-term influence of adjuvant breast radiotherapy on cognitive function in breast cancer patients treated with conservation therapy. Int J Clin Oncol, 24:68-77, 2019
5. Wada S, Inoguchi H, Sadahiro R, Matsuoka YJ, Uchitomi Y, Sato T, Shimada K, Yoshimoto S, Daiko H, Shimizu K. Preoperative Anxiety as a Predictor of Delirium in Cancer Patients: A Prospective Observational Cohort Study. World J Surg, 43:134-142, 2019
6. Kokubun H, Takigawa C, Miyano K, Uezono Y. A Novel Method for Determination of Methadone in the Serum by High-Performance Liquid Chromatography with Electrochemical Detection. Biol Pharm Bull, 41:649-651, 2018
7. Meguro Y, Miyano K, Hirayama S, Yoshida Y, Ishibashi N, Ogino T, Fujii Y, Manabe S, Eto M, Nonaka M, Fujii H, Ueta Y, Narita M, Sata N, Yada T, Uezono Y. Neuropeptide oxytocin enhances mu opioid receptor signaling as a positive allosteric modulator. J Pharmacol Sci, 137:67-75, 2018
8. Miyano K, Nonaka M, Uzu M, Ohshima K, Uezono Y. Multifunctional Actions of Ninjinyoeito, a Japanese Kampo Medicine: Accumulated Scientific Evidence Based on Experiments With Cells and Animal Models, and Clinical Studies. Front Nutr, 5:93, 2018
9. Kokubun Hideya, Honma Masashi, Miyano Kanako, Uezono Yasuhito. A Novel Method for Determination of Tapentadol in the Serum of Cancer Patients by High-performance Liquid Chromatography with Electrochemical Detection. Jpn. J. Pharm. Palliat. Care Sci, 11:131-133, 2018
10. Fujiwara M, Inagaki M, Nakaya N, Fujimori M, Higuchi Y, Kakeda K, Uchitomi Y, Yamada N. Smoking among adults with serious psychological distress: Analysis of anonymized data from a national cross-sectional survey in Japan. J Affect Disord, 239:131- 137, 2018
11. Inagaki M, Fujiwara M, Nakaya N, Fujimori M, Higuchi Y, Hayashibara C, So R, Kakeda K, Kodama M, Uchitomi Y, Yamada N. Low Cancer Screening Rates among Japanese People with Schizophrenia: A Cross-Sectional Study. Tohoku J Exp Med, 244:209-218, 2018
12. Zenda S, Yamaguchi T, Yokota T, Miyaji T, Mashiko T, Tanaka M, Yonemura M, Takeno M, Okano T, Kawasaki T, Nakamori Y, Ishii S, Shimada S, Kanamaru M, Uchitomi Y. Topical steroid versus placebo for the prevention of radiation dermatitis in head and neck cancer patients receiving chemoradiotherapy: the study protocol of J-SUPPORT 1602 (TOPICS study), a randomized double-blinded phase 3 trial. BMC Cancer, 18:873, 2018
13. Akechi T, Yamaguchi T, Uchida M, Imai F, Momino K, Katsuki F, Sakurai N, Miyaji T, Horikoshi M, Furukawa TA, Iwata H, Uchitomi Y. Smartphone problem-solving and behavioural activation therapy to reduce fear of recurrence among patients with breast cancer (SMartphone Intervention to LEssen fear of cancer recurrence: SMILE project): protocol for a randomised controlled trial. BMJ Open, 8:e024794, 2018
14. Yamada Y, Fujimori M, Shirai Y, Ninomiya H, Oka T, Uchitomi Y. Changes in Physicians' Intrapersonal Empathy After a Communication Skills Training in Japan. Acad Med, 93:1821-1826, 2018
15. Hamano J, Morita T, Mori M, Uchitomi Y. Talking About Palliative Sedation With the Family: Informed Consent vs. Assent and a Better Framework for Explaining Potential Risks. J Pain Symptom Manage, 56:e5-e8, 2018
16. Hayashibara C, Inagaki M, Fujimori M, Higuchi Y, Fujiwara M, Terada S, Okamura H, Uchitomi Y, Yamada N. Confidence in communicating with patients with cancer mediates the relationship between rehabilitation therapists' autistic-like traits and perceived difficulty in communication. Palliat Support Care, Jan 21:1-9, 2018
17. Ishida M, Onishi H, Morita T, Uchitomi Y, Shimizu M, Tsuneto S, Shima Y, Miyashita M. Communication Disparity Between the Bereaved and Others: What Hurts Them and What Is Unhelpful? A Nationwide Study of the Cancer Bereaved. J Pain Symptom Manage, 55:1061-1067.e1, 2018
18. Kako J, Morita T, Yamaguchi T, Kobayashi M, Sekimoto A, Kinoshita H, Ogawa A, Zenda S, Uchitomi Y, Inoguchi H, Matsushima E. Fan Therapy Is Effective in Relieving Dyspnea in Patients With Terminally Ill Cancer: A Parallel-Arm, Randomized Controlled Trial. J Pain Symptom Manage, 56:493-500, 2018
19. Hashimoto H, Abe M, Yanai T, Yamaguchi T, Zenda S, Uchitomi Y, Fukuda H, Mori M, Iwasa S, Yamamoto N, Ohe Y. Study protocol for J-SUPPORT 1604 (J-FORCE): a randomized, double blind, placebo-controlled Phase III study evaluating olanzapine (5 mg) plus standard triple antiemetic therapy for prevention of chemotherapy induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy. Jpn J Clin Oncol, 48:950-952, 2018
20. Terawaki K, Kashiwase Y, Uzu M, Nonaka M, Sawada Y, Miyano K, Higami Y, Yanagihara K, Yamamoto M, Uezono Y. Leukemia inhibitory factor via the Toll-like receptor 5 signaling pathway involves aggravation of cachexia induced by human gastric cancer-derived 85As2 cells in rats. Oncotarget, 9:34748-34764, 2018
Book
1. Uezono Y, Miyano K. Visceral hypersensitivity in functional dyspepsia (FD) - Therapeutic approaches to FD based on suppression of visceral hypersensitivity. In: Tominaga T, Kusunoki H (ed), Functional Dyspepsia: Evidences in Pathophysiology and Treatment, Singapore, Springer Singapore, pp 167-178, 2018
