Annual Report 2018
Clinical Laboratories
Kimihiko Kawamura, Motoi Miyakoshi, Masahiro Uchikawa, Susumu Wakai, Koji Ono, Hiroshi Yamakawa, Ryuzaburo Otake, Yoji Hashimoto, Yasuo Shibuki, Hiroki Kakishima, Tomohiro Nakatani, Koji Yamada, Rie Matsuo, Kazuya Tokita, Chiaki Ikeda, Susumu Ashikawa, Shuji Ota, Yukie Nakajima, Sachiko Kobayashi, Satoe Miyaki, Kumiko Nagasaki, Noriko Takahashi, Mizuho Fujima, Tomoe Ito, Kyoko Orihara, Kaori Ueki, Fumie Watanabe, Akino Kino, Takako Takada, Kyoko Osanai, Ruriko Machida, Asuka Matsunaga, Hiroshi Chigira, Go Sato, Sakiko Yoshimura, Yu Aruga, Saori Kobayashi, Kaori Yamaguchi, Ryoko Uegaki, Kensyo Kashiwaya, Saori Nakabayashi, Shingo Nakajima, Hideya Matsubayashi, Saeko Shirahama, Akiko Takayanagi, Mei Fukuhara, Kumi Nakatani, Momoko Kito, Moemi Kasane, Kazuhiro Yoshida, Kenta Takehara, Madoka Kondo, Yota Ikegami, Kana Katsuragi, Aisa Mizoguchi, Nao Iwashita, Mayu Takeno, Sakura Ishida, Yuri Kurosawa, Misato Tsubokura, Hitomi Nakamura, Megumi Masuda, Rana Goto, Iori Iida, Yuki Minagawa, Hiyori Yatsu, Yuri Tanaka, Kana Miyajima, Misaki Sato, Yoshiko Shibata, Mayumi Kamiya, Naomi Fujiki, Ritsuko Toyama, Chieko Nozawa, Kozaburo Endo, Shigeru Tamura, Mikako Kobayashi
Routine activities/research activities
Operations at the Department of Clinical Laboratories are organized into five sections: Clinical Laboratory Testing (urinalysis and other general testing, biochemistry, immunology, hematology, bacteriology, and gene testing), Blood Sampling, Transfusion and Cell Therapy Testing, Physiological Examination, and Pathological Examination. The Department has maintained the accreditation status under ISO 15189, which is the international standard defining the requirements for quality and competence in medical laboratories, after initial accreditation in September 2012 and successful completion of five cycles of surveillance and reassessment for renewal.
1) The Clinical Laboratory Testing Section has been responding to the increasing number of test orders by offering rapid and accurate test results to clinical departments, introducing state-of-the-art analytical equipment, and accommodating test items with high clinical significance. In immunology, we have been studying the clinical use of more specific tumor marker tests in collaboration with equipment and reagent manufacturers.
In hematology, the introduction of a flow cytometer with eight-color analysis capabilities contributed to the improvement of diagnostic accuracy for hematopoietic malignancies, and we also plan to strengthen the detection of minimal residual disease in various hematopoietic malignancies.
In bacteriology, mass spectroscopy is used for rapid identification of bacterial species and a gene analyzer has been introduced to speed up the detection of drug resistance genes, contributing to the appropriate use of antibiotics and the prevention of outbreaks.
In gene testing, we have been contributing to the use of cancer gene panel tests in actual clinical settings, plan to perform in-house tests using the next-generation sequencer (NGS), and develop a system for companion diagnostics.
2) The Blood Sampling Section has been contributing to the smooth operation of outpatient chemotherapy through blood sampling in early morning hours. We have also been responding appropriately to the increase in the number of outpatient blood samplings ordered by doctors, including the samplings for research purposes.
3) The Blood Transfusion and Cell Therapy Testing Section has been contributing to safety by determining the timing of peripheral stem cell collection using the measurement of hematopoietic progenitor cells with a multiparameter automated hematology analyzer and by quick reporting of the number of harvested cells using a flow cytometer immediately after peripheral stem cell collection.
4) The Physiological Examination Section has reduced the number of days patients spend on the waiting list and has been responding quickly to the requests for the tests related to clinical trials and the evaluation of cardiotoxicity of anticancer agents. A study is ongoing on a new method for the evaluation of the left ventricular systolic function.
5) The Pathological Examination Section has increased the number of automated immunostainers to cope with the everincreasing need for screening of patients for molecular target drugs. A study is ongoing toward the standardization of pre-analysis of pathology specimens.
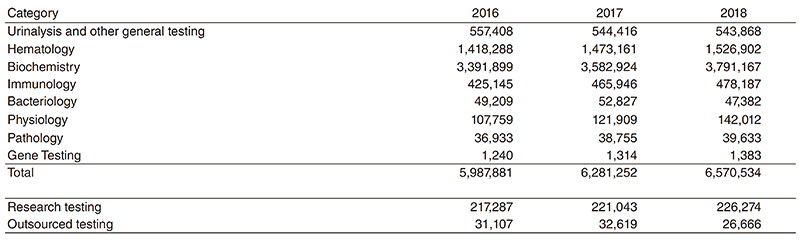
The number of clinical laboratory test orders has been increasing steadily in most sections. In 2018, the total number of clinical tests performed was 6,570,534, up 4.6% from the previous year (Table 1). The Clinical Laboratories Department is also moving forward with collaborations in clinical trials, translational research, and biobank projects.
Table 1. Number of clinical tests performed

Research results
The Clinical Laboratories is conducting basic and clinical studies concerning factors affecting the accuracy of laboratory tests.
Human resource training and education
Development and education of personnel are conducted according to the education and training protocol prescribed based on a quality management system (QMS) as per ISO 15189. With respect to routine work, all personnel are assessed annually for their capabilities through self-assessment and assessment by assessors using the check sheet for the understanding of work. Newly recruited technologists are trained using the check sheet for initial understanding to ensure the acquisition of work skills. In addition, newly recruited technologists undergo four-week training in blood sampling to learn the basic knowledge and skills including patient reception for blood sampling, handling specimens, and interaction with patients. With regard to academic activities, we host monthly conferences. In addition, we hold a workshop focusing on the quality and technical requirements of ISO 15189 four times a year, aiming to instill an understanding of QMS as per ISO 15189 among all personnel. As a rule, all workshop sessions are organized by the personnel of the Clinical Laboratories with the aim to improve their presentation skills. Each section sets its yearly goals for academic activities and conducts regular progress management as a means for systematically encouraging people to obtain certifications, contribute papers, and make presentations at academic conferences. Rehearsals are always performed before academic presentations to ensure the quality of presentations. With regard to student education, we maintain ongoing university internships, actively accept on-site practice trainees, and work to communicate the attraction of clinical testing operations.
Future prospects
As an ISO 15189-accredited testing facility, we guarantee the quality and capabilities meeting international standard and also promote cooperation in clinical trials and clinical studies. We expand the central blood drawing room to improve the efficiency of work related to blood sampling. We pursue standardization of the quality control system in cancer genomic medicine. We construct a cooperation system for cancer immunotherapy, such as chimeric antigen receptor T cell therapy (CAR-T). We strengthen the organizational capacity for performing ultrasound examination, aiming to improve qualitative diagnosis of tumors. We promote the training and use of highly specialized clinical laboratory technologists. We promote academic and research activities and disseminate information inside and outside of the country.
List of papers published in 2018
Journal
1. Ohashi-Nakatani K, Shibuki Y, Fujima M, Watanabe R, Yoshida A, Yoshida H, Matsumoto Y, Tsuchida T, Watanabe SI, Motoi N. Primary pulmonary meningioma: A rare case report of aspiration cytological features and immunohistochemical assessment. Diagn Cytopathol, 47:330-333, 2019
