Annual Report 2018
Division of Carcinogenesis and Cancer Prevention(Viral Carcinogenesis and Cancer Prevention Group)
Tohru Kiyono, Tomomi Nakahara, Takako Ishiyama, Katsuyuki Tanaka, Chiho Kohno, Yukimi Gotoh, Etsuko Kabasawa, Takashi Yugawa, Kenji Yamada, Shin-ichi Ohno, Yuki Inagawa
Introduction
Approximately 15% of human cancers have a viral etiology. The aim of the research in the Viral Carcinogenesis and Cancer Prevention Group is to elucidate the molecular mechanisms for carcinogenesis and viral persistence of human papillomavirus (HPV) infection in order to develop effective cancer prevention. Persistent infection of oncogenic HPVs is responsible for nearly 5% of all cancers including cervical, anal, vaginal, vulvar, penile, and head and neck cancers. The E6 and E7 proteins of oncogenic HPVs are known to inactivate the major tumor suppressors, p53 and retinoblastoma protein (pRB), respectively. By using an in vitro multistep carcinogenesis model, we study specific associations between oncogenic driver genes and pathological features of cancers with as well as without a viral etiology.
The Team and What We Do
To study molecular pathology of cancers with or without viral etiology, we are developing ex vivo carcinogenesis models by transducing genetic abnormalities frequently found in given cancers into their respective normal cells-of-origin. With our tissue culture models, we aim to (1) elucidate mechanisms of viral persistence of HPV infection and (2) analyze the association between driver mutations and cancer characteristics.
Research activities
1. HPV-induced carcinogenesis and its prevention
Eradication of persistent HPV infection can realize non-invasive treatment to prevent HPV associated cancers. To develop new strategies for viral elimination, it is critical to gain insight into the molecular mechanisms of the viral genome replication. HPV life cycle depends on the differentiation of the stratified epithelium. In basal cells, viral genomes are maintained as low copy number-episomes and expression of the viral genes is minimal. This latent state is thought to facilitate persistent infection by eluding host immune surveillance.
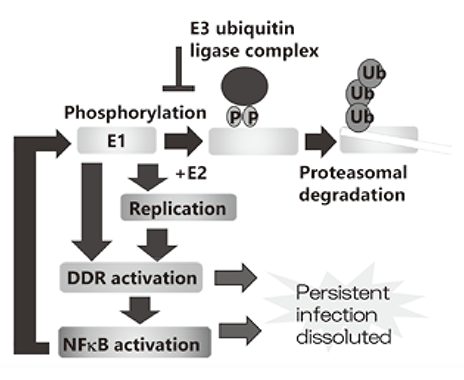
I. We established a tissue culture model which can recapitulate HPV life cycle and demonstrated that the viral helicase, E1 is dispensable for maintaining HPV episomes in basal cells. Subsequent studies indicated that E1 expression induces activation of NFΚB pathway and NFΚB functions as a negative feedback by facilitating phosphorylationdependent proteasomal degradation of E1. We propose that this negative feedback loop is a key molecular mechanism for the viral persistence by strictly limiting an E1 level in basal cells (Figure 1). We are currently investigating whether the dissolution of this feedback loop leads to elimination of virus infection.
Figure 1. E1-NFkB negative feedback loop

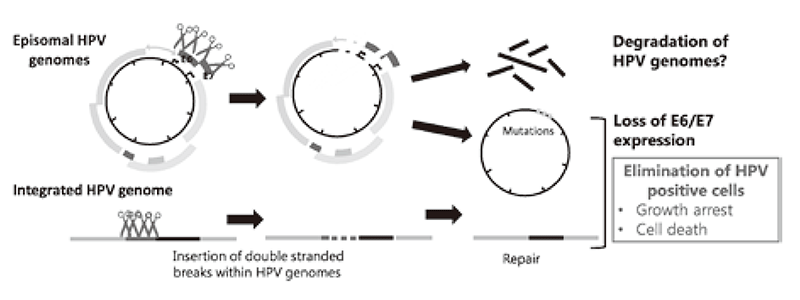
Genome editing technologies such as CRISPR/Cas9 made it possible to directly target the HPV genome. We also began to test the effectiveness of targeting HPV genomes by the CRISPR/Cas9 system to cure HPV infection as well as cervical cancer. (Figure 2).
Figure 2. Strategies for eradication of HPV genomes and/or HPV positive cells with CRISPR/Cas9

2. In vitro human carcinogenesis model
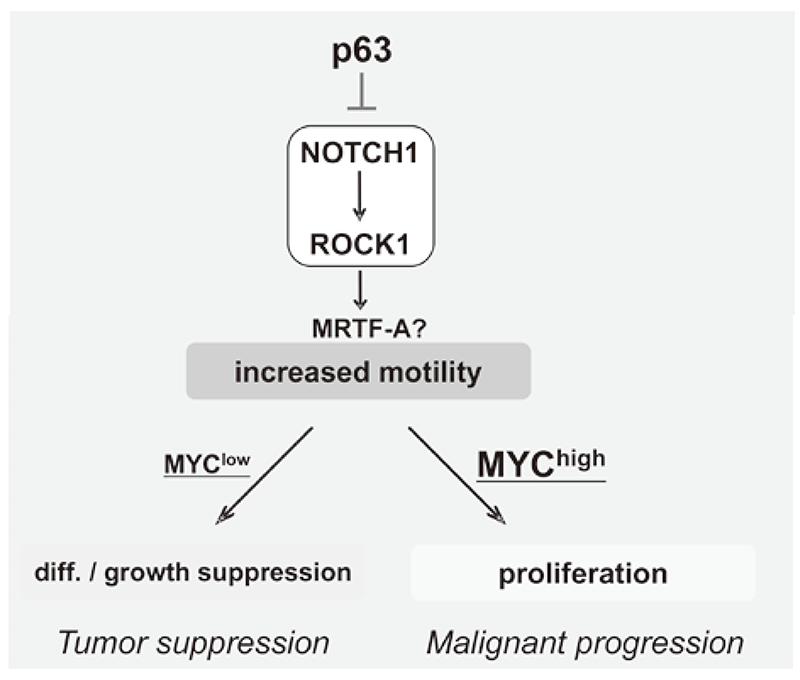
We have been studying the biological significance of ΔNp63α (hereafter p63) expression and the molecular basis underlying acquisition of malignant traits in squamous cell carcinomas (SCCs) of cervical and head and neck cancers. p63 plays an essential role in stem cell maintenance of stratified epithelium partly through transcriptional suppression of NOTCH1. Indeed, overexpression of p63 and inactivating mutation of NOTCH1 are frequently detected in human SCCs including examples in the cervix, lung, and head and neck. Paradoxically, loss of p63 is shown to be associated with metastasis appearance and poor prognosis in SCCs, although the underlying molecular mechanism and its functional relevance to carcinogenesis remains unclear as is the case with NOTCH1. We have demonstrated that loss of p63 induces terminal differentiation of normal keratinocytes through activation of the NOTCHROCK pathway. In MYC-overexpressing cells, however, knock-down of p63 conferred increased invasiveness through the NOTCH-ROCK pathway (Figure 3). Current studies focus on MRTF-A (MKL1) as a downstream effector in the NOTCH-ROCK pathway. Our goal is to develop novel strategies for diagnosis and molecularly targeted therapies against poorly differentiated SCCs based on their cancer biology.
Education
A PhD student from Thailand completed his PhD thesis in our laboratory and obtained a PhD from Khon Kaen University. A medical doctor from China whom we accepted as a trainee last year has enrolled in the PhD program of Keio University, Graduate School of Medicine and two Japanese undergraduate students who started last year have enrolled in Master programs (Kitasato University and Tokyo Medical and Dental University). All three of them continue to work in our laboratory as graduate students this year.
Future prospects
We are seeing promising results from development of HPV targeting gene therapy with the CRISPR/Cas9 system. Based on our findings that uncover a molecular mechanism for viral persistence, we are developing a new approach for eradication of HPV infection. The in vitro carcinogenesis model for any given cancer can be established in vitro with our techniques. These models will be useful for preclinical assessment of new cancer therapies.
List of papers published in 2018
Journal
1. Inoue M, Kajiwara K, Yamaguchi A, Kiyono T, Samura O, Akutsu H, Sago H, Okamoto A, Umezawa A. Autonomous trisomic rescue of Down syndrome cells. Lab Invest, 2019
2. Yoshida M, Taguchi A, Kawana K, Ogishima J, Adachi K, Kawata A, Nakamura H, Sato M, Fujimoto A, Inoue T, Tomio K, Mori M, Nagamatsu T, Arimoto T, Koga K, Hiraike OW, Oda K, Kiyono T, Osuga Y, Fujii T. Intraperitoneal neutrophils activated by KRAS-induced ovarian cancer exert antitumor effects by modulating adaptive immunity. Int J Oncol, 53:1580-1590, 2018
3. Wakae K, Nishiyama T, Kondo S, Izuka T, Que L, Chen C, Kase K, Kitamura K, Mohiuddin M, Wang Z, Ahasan MM, Nakamura M, Fujiwara H, Yoshizaki T, Hosomochi K, Tajima A, Nakahara T, Kiyono T, Muramatsu M. Keratinocyte differentiation induces APOBEC3A, 3B, and mitochondrial DNA hypermutation. Sci Rep, 8:9745, 2018
4. Nakamura K, Nakayama K, Ishikawa N, Ishikawa M, Sultana R, Kiyono T, Kyo S. Reconstitution of high-grade serous ovarian carcinoma from primary fallopian tube secretory epithelial cells. Oncotarget, 9:12609-12619, 2018
5. Kasahara K, Aoki H, Kiyono T, Wang S, Kagiwada H, Yuge M, Tanaka T, Nishimura Y, Mizoguchi A, Goshima N, Inagaki M. EGF receptor kinase suppresses ciliogenesis through activation of USP8 deubiquitinase. Nat Commun, 9:758, 2018
6. Inoko A, Yano T, Miyamoto T, Matsuura S, Kiyono T, Goshima N, Inagaki M, Hayashi Y. Albatross/FBF1 contributes to both centriole duplication and centrosome separation. Genes Cells, 23:1023-1042, 2018
7. Gouko R, Onuma M, Eitsuka T, Katayama M, Takahashi K, Nakagawa K, Inoue-Murayama M, Kiyono T, Fukuda T. Efficient immortalization of cells derived from critically endangered Tsushima leopard cat (Prionailurus bengalensis euptilurus) with expression of mutant CDK4, Cyclin D1, and telomerase reverse transcriptase. Cytotechnology, 70:1619-1630, 2018
8. Fukuda T, Eitsuka T, Donai K, Kurita M, Saito T, Okamoto H, Kinoshita K, Katayama M, Nitto H, Uchida T, Onuma M, Sone H, Inoue-Murayama M, Kiyono T. Expression of human mutant cyclin dependent kinase 4, Cyclin D and telomerase extends the life span but does not immortalize fibroblasts derived from loggerhead sea turtle (Caretta caretta). Sci Rep, 8:9229, 2018
