Annual Report 2019
Division of Cancer Immunology
Hiroyoshi Nishikawa, Shohei Koyama, Yoshihiro Ohue, Yosuke Togashi, Takuma Irie, Kota Itahashi, Yasuko Tada, Rika Fujii, Sho Watanabe, Tomomi Morioka, Miki Yamazaki, Chika Sakai, Eri Sugiyama, Shota Fukuoka, Koji Inamori, Kumiko Umemoto, Akihito Kawazoe, Daiki Sato, Takao Fujisawa, Nobuhiko Yamauchi, Junichiro Yuda, Yuuki Ohara, Shogo Kumagai, Tokiyoshi Tanegashima, Joji Nagasaki, Ryo Ariyasu, Ayaka Tsuge, Yi-Tzu Lin, Tamiyo Kobayashi, Sho Isoyama, Sayoko Shinya, Hidenori Kasahara, Konomi Onagawa, Tomoka Takaku, Miyuki Nakai, Megumi Takemura, Megumi Hoshino, Kumiko Yoshida, Mizuho Kikuchi, Yoko Ohira, Sayuri Yoshimatsu, Yumiko Takamatsu
Introduction
The recent success of cancer immunotherapy, particularly immune checkpoint inhibitors (ICIs), makes it a key strategy for cancer treatment. Among patients receiving ICIs, while some experience long-lasting clinical benefits, more than half fail to exhibit clinical any benefits. Moreover, some patients experience recurrence after initially responding to such immunotherapy. It is therefore urgently required to identify predictive biomarkers, and to develop optimal combination treatments with immunotherapies for individual patients.
The Team and What We Do
In our laboratory, cancer cells and immune cells in the tumor microenvironment or peripheral blood are being investigated using several techniques, including multi-color flow/masscytometry, RNA sequencing and whole exome sequencing by collaboration with clinical departments (Figure 1). Real-time immune monitoring in cancer patients undergoing treatment has been established, through which we have had collaborations with pharmaceutical companies in their clinical trials. Especially, regulatory T cells (Treg), which have a strong immune suppressive function and are known to be a critical component to inhibit anti-tumor immunity, are analyzed with our original method.
Furthermore, with the findings from clinical samples analyses, we also reverse-translate them into basic research to elucidate the essential mechanisms of immune responses to cancer.
Figure 1. We are investigating the dynamic immune state in cancer patients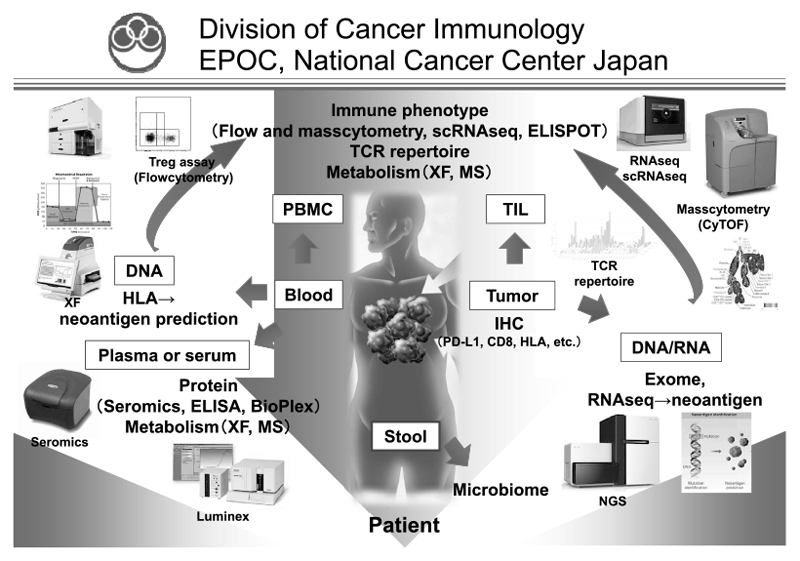

Research activities
When the data from immune profiling in a tumor microenvironment were integrated with genomic analyses in non-small cell lung cancer (NSCLC), we found that EGFR mutation positively recruited Treg into the tumor as compared to wild-type NSCLC (Sugiyama E et al., Sci Immunology, 2020). We also found that Rho-A mutation led to a Treg-rich immune microenvironment in gastric cancer through fatty acid production (Kumagai S et al., Immunity, in press) (Figure 2).
Using our real-time immune monitoring system, we addressed kinetic changes of immune status in a tumor microenvironment after cancer therapies including ICIs and cytotoxic reagents. We found a reduction of activated Treg cells by a first-in-human clinical reagent using our real-time immune monitoring system, proposing a novel effect of this drug.
Furthermore, as a promising predictive biomarker, a combination of two factors, PD-1 positivity in CD8 and effector Treg (eTreg), have been identified in patients treated with immune checkpoint inhibitors. A subset of patients in Group R: PD-1+CD8+TILs>40% and PD-1 expression ratio CD8T/eTreg>1 showed a much better outcome of ICI treatment in patients with lung cancer, gastric cancer and melanoma (Figure 3).
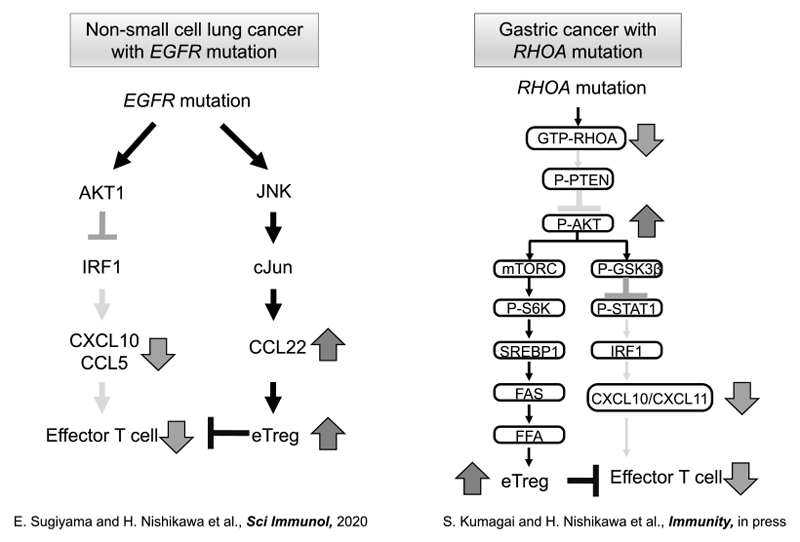
Figure 3. Optimizing cancer immunotherapies based on biomarkers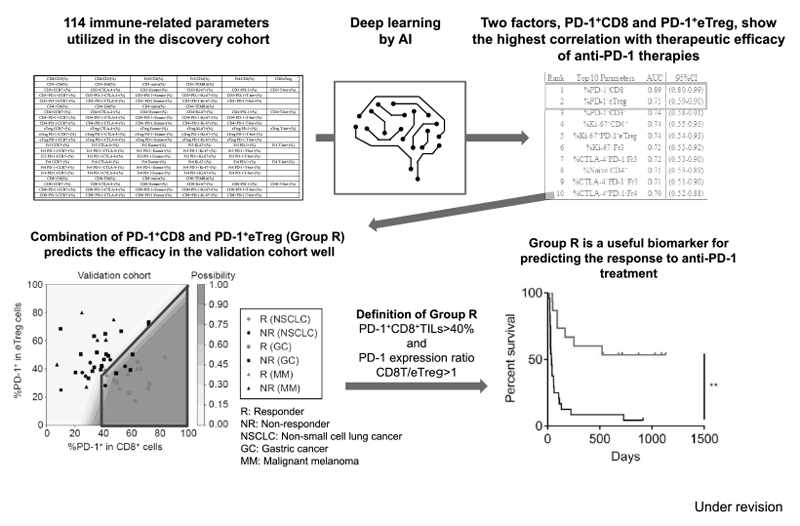

Clinical trials
We have collaborated with several pharmaceutical companies in their clinical trials to elucidate biomarkers for predicting the therapeutic response and to evaluate immune status in a tumor microenvironment using our real-time immune monitoring.
Education
Many graduate school students are trained in our division, and young residents in the National Cancer Center East are also trained to become physician scientists. After finishing their PhD course, some of students continue to study cancer immunology abroad.
Future prospects
ICIs have undoubtedly opened a new era in cancer treatment. However, we still have several issues to address, including acquired resistance and suppressive immune modulation by somatic mutations in cancer cells, in order to overcome the drawbacks of ICIs. In order to solve these questions, immunological analyses using clinical specimens are essential. Therefore, it is important to develop better therapeutic strategies combining ICIs with other anti-cancer drugs and to identify biomarkers which can stratify responders from non- responders via clarifying the immune suppressive networks in TME
List of papers published in 2019
Journal
1. Sugiyama E, Togashi Y, Takeuchi Y, Shinya S, Tada Y, Kataoka K, Tane K, Sato E, Ishii G, Goto K, Shintani Y, Okumura M, Tsuboi M, Nishikawa H. Blockade of EGFR improves responsiveness to PD-1 blockade in EGFR-mutated non-small cell lung cancer. Sci Immunol, 5:eaav3937. doi: 10.1126/sciimmunol.aav3937, 2020
2. Tanaka A, Nishikawa H, Noguchi S, Sugiyama D, Morikawa H, Takeuchi Y, Ha D, Shigeta N, Kitawaki T, Maeda Y, Saito T, Shinohara Y, Kameoka Y, Iwaisako K, Monma F, Ohishi K, Karbach J, Jäger E, Sawada K, Katayama N, Takahashi N, Sakaguchi S. Tyrosine kinase inhibitor imatinib augments tumor immunity by depleting effector regulatory T cells. J Exp Med, 217:pii: e20191009. doi: 10.1084/jem.20191009, 2020
3. Sasaki A, Nakamura Y, Togashi Y, Kuno H, Hojo H, Kageyama S, Nakamura N, Takashima K, Kadota T, Yoda Y, Mishima S, Sawada K, Kotani D, Kawazoe A, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Yano T, Kobayashi T, Akimoto T, Nishikawa H, Shitara K. Enhanced tumor response to radiotherapy after PD-1 blockade in metastatic gastric cancer. Gastric Cancer, 2020
4. Watanabe S, Shimomura A, Kubo T, Sekimizu M, Seo T, Watanabe SI, Kawai A, Yamamoto N, Tamura K, Kohno T, Ichikawa H, Yoshida A. BRAF V600E mutation is a potential therapeutic target for a small subset of synovial sarcoma. Mod Pathol, 2020
5. Mizuno T, Horinouchi H, Watanabe S, Sato J, Morita R, Murakami S, Goto Y, Kanda S, Fujiwara Y, Yamamoto N, Ohe Y. Number of metastatic organs negatively affects the treatment sequence in patients with EGFR-TKI failure. Thorac Cancer, 11:1038-1044, 2020
6. Minoura K, Abe K, Maeda Y, Nishikawa H, Shimamura T. Model-based cell clustering and population tracking for time-series flow cytometry data. BMC Bioinformatics, 20:633, 2019
7. Tokunaga A, Sugiyama D, Maeda Y, Warner AB, Panageas KS, Ito S, Togashi Y, Sakai C, Wolchok JD, Nishikawa H. Selective inhibition of low-affinity memory CD8+ T cells by corticosteroids. J Exp Med, 216:2701-2713, 2019
8. Doi T, Muro K, Ishii H, Kato T, Tsushima T, Takenoyama M, Oizumi S, Gemmoto K, Suna H, Enokitani K, Kawakami T, Nishikawa H, Yamamoto N. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin Cancer Res, 25:6614-6622, 2019
9. Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci, 110:2080-2089, 2019
10. Datta M, Coussens LM, Nishikawa H, Hodi FS, Jain RK. Reprogramming the Tumor Microenvironment to Improve Immunotherapy: Emerging Strategies and Combination Therapies. Am Soc Clin Oncol Educ Book, 39:165-174, 2019
11. Tanegashima T, Togashi Y, Azuma K, Kawahara A, Ideguchi K, Sugiyama D, Kinoshita F, Akiba J, Kashiwagi E, Takeuchi A, Irie T, Tatsugami K, Hoshino T, Eto M, Nishikawa H. Immune Suppression by PD-L2 against Spontaneous and Treatment-Related Antitumor Immunity. Clin Cancer Res, 25:4808-4819, 2019
12. Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, Morikawa H, Kawazoe A, Kinoshita T, Shitara K, Sakaguchi S, Nishikawa H. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A, 116:9999-10008, 2019
13. Kochin V, Nishikawa H. <Editors' Choice> Meddling with meddlers: curbing regulatory T cells and augmenting antitumor immunity. Nagoya J Med Sci, 81:1-18, 2019
