Annual Report 2019
Division of Developmental Therapeutics
Yasuhiro Matsumura, Masahiro Yasunaga, Yoshikatsu Koga, Hiroki Takashima, Ryo Tsumura, Hirobumi Fuchigami, Takahiro Anzai, Boran Osman, Shigehiro Koganemaru, Kenji Takashima, Takuya Shiraishi, Kaori Hayashi, Yoko Omichi, Makoto Wakatsuki, Tamaki Hirakawa, Nozomi Iwata, Daisuke Kamakura, Shiqi Yang, Nakayama, Mamiko Shimada, Shinji Saijo, Shingo Hanaoka
Introduction
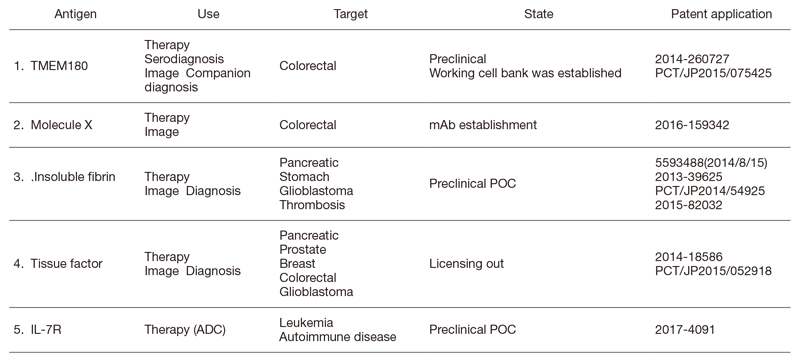
The Division of Developmental Therapeutics has been involved in basic research on drug delivery systems (DDSs) and antibody therapeutics including monoclonal antibody (mAb) development, antibody drug conjugates (ADCs), radioimmunotherapy (RIT), bispecific antibodies (BsAbs), and the mAb conjugated micelle system. We have applied for patents for all mAbs established in our division (Table 1). We have developed a unique type of ADC, namely CAST (cancer stromal targeting) therapy, which utilizes dense stroma as a scaffold for drug delivery. In addition to the research work, we are operating the Japan Clinical Oncology Group (JCOG) Tumor Repository.
The Team and What We Do
- Development of antibody drugs and their deployment to pharmaceutical companies
- Conducting translational research utilizing mass spectrometry
- Operation of the JCOG Tumor Repository
Research activities
1. DDS in cancer chemotherapy
Tumor-targeted delivery of therapeutic agents is a longstanding pharmacological goal to improve the treatment selectivity and the therapeutic index. Most scientists have sought to use “active” receptor-mediated tumor-targeting systems. However, the “passive” targeting afforded by the “Enhanced Permeability and Retention (EPR) effect” provides a versatile and non-saturable approach for tumor-selective delivery. Polymeric micelles are ideally suited to exploit the EPR effect and have been used for the delivery of a range of anticancer drugs in preclinical and clinical studies. Anti-TF mAb conjugated micelles incorporating epirubicin also exerted a potent antitumor effect in a human pancreatic cancer model.
2. Next-generation antibody therapeutics
Although clinical applications of antibody therapeutics and next-generation antibody therapeutics centered on ADCs have progressed, they have not been applied to refractory cancers such as pancreatic and diffuse gastric cancers. We reported that drug delivery could be disturbed by dense stroma in these tissues (i.e., the tumor stromal barrier). To overcome the tumor stromal barrier, we developed a unique strategy in which the cancer stromal targeting (CAST) therapy by cytotoxic immunoconjugate bound to the collagen 4, tissue factor (TF), or fibrin network in the tumor stroma. From there the payload released gradually and distributed throughout the tumor, resulting in the arrest of tumor growth due to induced damage to tumor cells and tumor vessels. Molecular imaging is very useful for visually evaluating antibody delivery. In addition, refractory or remnant tumor cells themselves are likely to be resistant to general chemotherapy. Alpha emitters with high-energy and linear-energy transfer, which exert greater cytotoxicity, could be used for the treatment of chemoresistant-refractory cancer. Hence, we are pursuing pharmaceutical research and development of ADCs and RIT in collaboration with professionals in various research fields. We are also developing T cell-dependent bispecific antibodies which can eliminate tumor cells independent of MHC engagement and are expected to be a novel breakthrough immunotherapy against refractory cancer. Furthermore, we have found various cell surface molecules specific to colorectal cancer and created mAbs that correspond to those molecules. Among them, we are developing anti-TMEM 180 mAb for clinical application.
3. Advanced mass spectrometry and bioinformatics systems
Mass spectrometry studies allow us to visualize payloads released from ADCs and observe the signature of drug-induced cell injury by monitoring changes in the biomolecules and metabolites influenced by them. Such approaches would be helpful for elucidating the bystander effect and immunogenic cell death (ICD), which lie at the cutting edge of ADC research. Therefore, we are able to evaluate the mechanism of action (MOA), pharmacokinetics (PK), and pharmacodynamics (PD) of new drugs utilizing advanced mass spectrometry and bioinformatics systems. They are also useful for discovering target molecules for the development of therapeutic antibodies.
Education
1) Doctoral students
Graduate School of Frontier Sciences, the University of Tokyo: two students
Keio University School of Medicine: one student
Nihon University School of Medicine: one student
2) Master course students
Graduate School of Frontier Sciences, the University of Tokyo: five students
Future prospects
We are developing ADCs and RIT as armed therapeutic antibodies, as well as BsAbs that can activate T cells directly. In addition to our regular research approach using DDSs and molecular imaging, we are also aggressively incorporating cell biological technology. Overall, we are taking a unique drug discovery approach based on host factors as well as the drugs themselves. Our advanced mass spectrometry and bioinformatics analysis can support effective translation of preclinical research to humans. Our cutting-edge research, such as the co-clinical trial project and DDSs based on nanotechnology, will also promote development of new drugs through interdisciplinary fusion and industry–academia collaboration.
List of papers published in 2019
Journal
1. Numasawa K, Hanaoka K, Saito N, Yamaguchi Y, Ikeno T, Echizen H, Yasunaga M, Komatsu T, Ueno T, Miura M, Nagano T, Urano Y. A Fluorescent Probe for Rapid, High-Contrast Visualization of Folate-Receptor-Expressing Tumors In?Vivo. Angew Chem Int Ed Engl, 59:6015-6020, 2020
2. Takashima H, Koga Y, Tsumura R, Yasunaga M, Tsuchiya M, Inoue T, Negishi E, Harada M, Yoshida S, Matsumura Y. Reinforcement of antitumor effect of micelles containing anticancer drugs by binding of an anti-tissue factor antibody without direct cytocidal effects. J Control Release, 323:138-150, 2020
3. Yasunaga M. Antibody therapeutics and immunoregulation in cancer and autoimmune disease. Semin Cancer Biol, 64:1-12, 2020
4. Yagi H, Yagi-Utsumi M, Honda R, Ohta Y, Saito T, Nishio M, Ninagawa S, Suzuki K, Anzai T, Kamiya Y, Aoki K, Nakanishi M, Satoh T, Kato K. Improved secretion of glycoproteins using an N-glycan-restricted passport sequence tag recognized by cargo receptor. Nat Commun, 11:1368, 2020
5. Yasunaga M, Saijou S, Hanaoka S, Anzai T, Tsumura R, Matsumura Y. Significant antitumor effect of an antibody against TMEM180, a new colorectal cancer-specific molecule. Cancer Sci, 110:761-770, 2019
6. Koganemaru S, Kuboki Y, Koga Y, Kojima T, Yamauchi M, Maeda N, Kagari T, Hirotani K, Yasunaga M, Matsumura Y, Doi T. U3-1402, a Novel HER3-Targeting Antibody-Drug Conjugate, for the Treatment of Colorectal Cancer. Mol Cancer Ther, 18:2043-2050, 2019
7. Tsumura R, Manabe S, Takashima H, Koga Y, Yasunaga M, Matsumura Y. Evaluation of the antitumor mechanism of antibody-drug conjugates against tissue factor in stroma-rich allograft models. Cancer Sci, 110:3296-3305, 2019
8. Manabe S, Yamaguchi Y, Matsumoto K, Fuchigami H, Kawase T, Hirose K, Mitani A, Sumiyoshi W, Kinoshita T, Abe J, Yasunaga M, Matsumura Y, Ito Y. Characterization of Antibody Products Obtained through Enzymatic and Nonenzymatic Glycosylation Reactions with a Glycan Oxazoline and Preparation of a Homogeneous Antibody-Drug Conjugate via Fc N-Glycan. Bioconjug Chem, 30:1343-1355, 2019
9. Sugyo A, Aung W, Tsuji AB, Sudo H, Takashima H, Yasunaga M, Matsumura Y, Saga T, Higashi T. Anti?tissue factor antibody?mediated immuno?SPECT imaging of tissue factor expression in mouse models of pancreatic cancer. Oncol Rep, 41:2371-2378, 2019
10. Anzai T, Matsumura Y. Topological analysis of TMEM180, a newly identified membrane protein that is highly expressed in colorectal cancer cells. Biochem Biophys Res Commun, 520:566-572, 2019
11. Koga Y, Ochiai A. Systematic Review of Patient-Derived Xenograft Models for Preclinical Studies of Anti-Cancer Drugs in Solid Tumors. Cells, 8:418, 2019
Book
1. Yasunaga M, Manabe S, Matsumura Y. CAST therapy. In: Matsumura Y, Tarin D (eds), Cancer Drug Delivery Systems Based on the Tumor Microenvironment, 東京, Springer Japan, pp 269-288, 2019
2. Matsumura Y. Cancer and Blood Coagulation. In: Matsumura Y, Tarin D (eds), Cancer Drug Delivery Systems Based on the Tumor Microenvironment, 東京, Springer Japan, pp 23-40, 2019
3. Koga Y, Tsumura R, Matsumura Y. Preclinical Studies of ADC Therapy for Solid Tumors. In: Matsumura Y, Tarin D (eds), Cancer Drug Delivery Systems Based on the Tumor Microenvironment, Tokyo, Springer Japan, pp 125-154, 2019
