Annual Report 2019
Department of Head and Neck Medical Oncology
Makoto Tahara, Susumu Okano, Takao Fujisawa, Yuri Ueda, Kazue Ito
Introduction
The Department of Head and Neck Medical Oncology is engaged in the clinical management of patients with head and neck cancer (HNC), and research into anticancer drugs for the treatment of HNC.
Our missions are to: 1) provide the best evidence-based treatment; 2) promote the importance of supportive care in the treatment of patients with HNC; 3) facilitate the timely approval of new drugs by active participation in global clinical trials to eliminate the drug lag; 4) develop cutting-edge treatments; and 5) train experts in head and neck medical oncology.
The Team and What We Do
Our department consists of three physicians, one senior resident and one resident. We manage the treatment of HNC patients who receive anticancer drugs. An estimated 60% of HNC patients require a multidisciplinary approach, including surgery, radiotherapy, and chemotherapy. Given the increasing complexity of the management of HNC, recommended treatment for patients who are referred to our institution is decided at a weekly tumor board attended by a multidisciplinary team.
A total of 415 patients were referred to our department from April 2019 to March 2020 (Table 1, Table 2). The outpatient service of our department is available from Monday to Friday. We carefully follow patients during and after treatment and provide palliative chemotherapy as an outpatient service.
Table 1. Number of patients to according site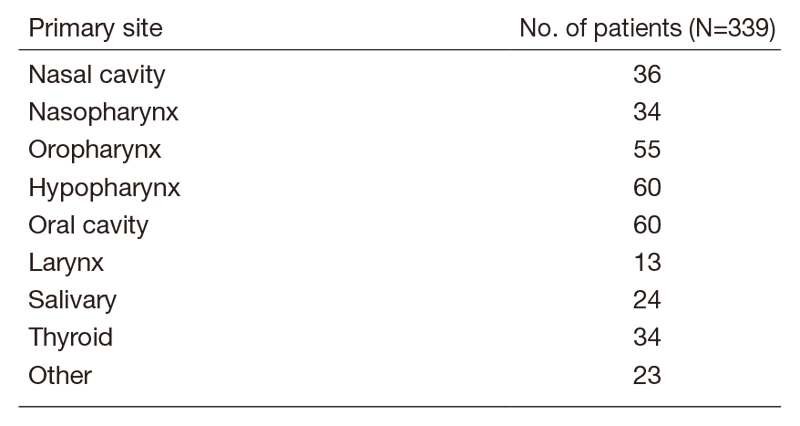

Table 2. No. of patients according to procedure

Research activities
Our research activity has focused on three areas, the development of new treatments in clinical trials for HNC, biomarker analysis in HNC and retrospective analysis of management of treatment for HNC.
We assessed the combination of paclitaxel, carboplatin, and cetuximab (PCE) as induction chemotherapy (IC) for unresectable LA-SCCHN. A total of 35 patients were enrolled. Of 35 patients, 34 (97%) completed IC and 32 received CRT. The %CRT completion was 96.9%, which exceeded the prespecified boundary of 84%. Mean cumulative dose and RDI of CDDP in CRT was 232.5 mg/m2 and 100%, respectively. Response rate was 88.6% by IC and 93.8% in the CRT phase. Two-year overall survival was 83.5%. Main grade 3 toxicities included neutropenia (11.4%) and skin rash (5.7%) during IC; and oral mucositis (31.3%) and neutropenia (12.5%) during CRT. No grade 4 toxicity or treatment-related death was seen. PCE as IC was feasible, with promising efficacy and no effect on compliance with subsequent CRT in unresectable LA-SCCHN.
We investigated the impact of lung metastases on survival in patients from SELECT, which was a randomized phase 3 study of lenvatinib versus placebo in patients who had radioiodine-refractory differentiated thyroid cancer (RR-DTC). Lenvatinib-treated population distributions per baseline lung-metastases subgroup were: any lung metastases (target/nontarget lesions; n=226); and by sum of diameters of target lung lesions, ≥1.0 cm (n=199), ≥1.5 cm (n=150), ≥2.0 cm (n=94), and <2.0 cm (n=105). In patients with any lung metastases, no statistically significant difference in OS was observed between treatment arms (HR: 0.76; 95%CI: 0.57-1.01; P=0.0549). Median OS for lung metastases of ≥1.0 cm was 44.7 months (lenvatinib) versus 33.1 months (placebo) (HR: 0.63; 95%CI: 0.47-0.85; P=0.0025). OS was significantly prolonged with lenvatinib versus placebo among patients with lung metastases of ≥1.0 cm, ≥1.5 cm, ≥2.0 cm, and <2.0 cm. ECOG PS (0 vs. ≥1) (P<0.0001) and age group (≤65 years vs. >65 years) (P=0.0243) had a significant impact on OS. Lenvatinib treatment resulted in longer OS in patients with lung metastases of ≥1.0 cm versus placebo, even with the 89% cross-over rate from placebo. Early initiation of lenvatinib may improve outcomes in patients with RR-DTC and lung metastases of ≥1.0 cm.
Clinical trials
The following investigator-initiated clinical trials are ongoing: 1) a phase 2 study of lenvatinib for anaplastic thyroid cancer, 2) a cohort study exploring the effect of lenvatinib on differentiated thyroid cancer, 3) a phase 2 study of combination with nivolumab plus lenvatinib for unresectable anaplastic thyroid cancer, and 4) a phase 2 study of darolutamide for androgen receptor positive recurrent or metastatic salivary gland carcinoma.
To facilitate the timely approval of new drugs and eliminate the drug lag, we have also participated in the global phase trials including immune-checkpoint inhibitors.
Education
We educate not only medical staff in our institute but also outside our institute by conducting the following education program: Seminar of the Japan Society of Supportive Care for Patients with HNC. Furthermore, our department is accepting trainees all the time.
Future prospects
We hope that ongoing or planned clinical trials will change the standard of care for HNC and our biomarker analysis will lead to the development of new treatment strategies. Our education program will increase the number of medical oncologists who take charge of treatment for HNC, leading to improving patient’s quality of survival.
List of papers published in 2019
Journal
1. Enokida T, Ogawa T, Homma A, Okami K, Minami S, Nakanome A, Shimizu Y, Maki D, Ueda Y, Fujisawa T, Motegi A, Ohkoshi A, Taguchi J, Ebisumoto K, Nomura S, Okano S, Tahara M. A multicenter phase II trial of paclitaxel, carboplatin, and cetuximab followed by chemoradiotherapy in patients with unresectable locally advanced squamous cell carcinoma of the head and neck. Cancer Med, 9:1671-1682, 2020
2. Naito Y, Mishima S, Akagi K, Igarashi A, Ikeda M, Okano S, Kato S, Takano T, Tsuchihara K, Terashima K, Nishihara H, Nishiyama H, Hiyama E, Hirasawa A, Hosoi H, Maeda O, Yatabe Y, Okamoto W, Ono S, Kajiyama H, Nagashima F, Hatanaka Y, Miyachi M, Kodera Y, Yoshino T, Taniguchi H. Japan society of clinical oncology/Japanese society of medical oncology-led clinical recommendations on the diagnosis and use of tropomyosin receptor kinase inhibitors in adult and pediatric patients with neurotrophic receptor tyrosine kinase fusion-positive advanced solid tumors, cooperated by the Japanese society of pediatric hematology/oncology. Int J Clin Oncol, 25:403-417, 2020
3. Horinouchi A, Suzuki S, Enokida T, Kamata H, Kaneko A, Matsuyama C, Tahara M. Grade 3 infusion-related reaction because of cetuximab administered with 5-fluorouracil and cisplatin chemotherapy for a recurrent and metastatic head and neck cancer patient who received chlorpheniramine 5mg, dexamethasone 13.2mg, and aprepitant 125mg premedication. Eur J Oncol Pharm, 3:e21 , 2020
4. Zenda S, Ota Y, Kiyota N, Okano S, Fujii M, Kitamura M, Takahashi S, Ueda T, Monden N, Yamanaka T, Tahara M. A Multicenter Phase II Trial of Docetaxel, Cisplatin, and Cetuximab (TPEx) Followed by Cetuximab and Concurrent Radiotherapy for Patients With Local Advanced Squamous Cell Carcinoma of the Head and Neck (CSPOR HN01: ECRIPS Study). Front Oncol, 9:6, 2019
5. Takahashi S, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, Fukuda N, Sasaki T, Suzuki T, Ikezawa H, Dutcus CE, Tahara M. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol, 15:717-726, 2019
6. Tahara M, Brose MS, Wirth LJ, Suzuki T, Miyagishi H, Fujino K, Dutcus CE, Gianoukakis A. Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine-refractory differentiated thyroid cancer. Eur J Cancer, 106:61-68, 2019
7. Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V, Adelstein D, Van Gestel D, Vermorken JB. Low-Dose vs. High-Dose Cisplatin: Lessons Learned From 59 Chemoradiotherapy Trials in Head and Neck Cancer. Front Oncol, 9:86, 2019
8. Okano S, Enokida T, Onoe T, Ota Y, Motegi A, Zenda S, Akimoto T, Tahara M. Induction TPF chemotherapy followed by CRT with fractionated administration of cisplatin in patients with unresectable locally advanced head and neck cancer. Int J Clin Oncol, 24:789-797, 2019
9. Matsuzuka T, Kiyota N, Mizusawa J, Akimoto T, Fujii M, Hasegawa Y, Iwae S, Monden N, Matsuura K, Onozawa Y, Hayashi R, Tahara M. Clinical impact of cachexia in unresectable locally advanced head and neck cancer: supplementary analysis of a phase II trial (JCOG0706-S2). Jpn J Clin Oncol, 49:37-41, 2019
10. Monden N, Asakage T, Kiyota N, Homma A, Matsuura K, Hanai N, Kodaira T, Zenda S, Fujii H, Tahara M, Yokota T, Akimoto T, Iwae S, Onitsuka T, Ogawa T, Okano S, Takahashi S, Shimizu Y, Yonezawa K, Hayashi R. A review of head and neck cancer staging system in the TNM classification of malignant tumors (eighth edition). Jpn J Clin Oncol, 49:589-595, 2019
11. Haddad R, Guigay J, Keilholz U, Clement PM, Fayette J, de Souza Viana L, Rolland F, Cupissol D, Geoffrois L, Kornek G, Licitra L, Melichar B, Ribaldo Nicolau U, Rauch D, Zanetta-Devauges S, Cohen EEW, Machiels JP, Tahara M, Vermorken J, Geng Y, Zografos E, Gauler T. Afatinib as second-line treatment in patients with recurrent/metastatic squamous cell carcinoma of the head and neck: Subgroup analyses of treatment adherence, safety and mode of afatinib administration in the LUX-Head and Neck 1 trial. Oral Oncol, 97:82-91, 2019
12. Ferris RL, Licitra L, Fayette J, Even C, Blumenschein G Jr, Harrington KJ, Guigay J, Vokes EE, Saba NF, Haddad R, Ramkumar S, Russell J, Brossart P, Tahara M, Colevas AD, Concha-Benavente F, Lynch M, Li L, Gillison ML. Nivolumab in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: Efficacy and Safety in CheckMate 141 by Prior Cetuximab Use. Clin Cancer Res, 25:5221-5230, 2019
13. Burtness B, Haddad R, Dinis J, Trigo J, Yokota T, de Souza Viana L, Romanov I, Vermorken J, Bourhis J, Tahara M, Martins Segalla JG, Psyrri A, Vasilevskaya I, Nangia CS, Chaves-Conde M, Kiyota N, Homma A, Holeckova P, Del Campo JM, Asarawala N, Nicolau UR, Rauch D, Even C, Wang B, Gibson N, Ehrnrooth E, Harrington K, Cohen EEW. Afatinib vs Placebo as Adjuvant Therapy After Chemoradiotherapy in Squamous Cell Carcinoma of the Head and Neck: A Randomized Clinical Trial. JAMA Oncol, 5:1170-1180, 2019
14. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesía R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Hong RL, González Mendoza R, Roy A, Zhang Y, Gumuscu B, Cheng JD, Jin F, Rischin D. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet, 394:1915-1928, 2019
15. Haddad R, Concha-Benavente F, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Kasper S, Vokes EE, Worden F, Saba NF, Tahara M, Jayaprakash V, Lynch M, Li L, Gillison ML, Harrington KJ, Ferris RL. Nivolumab treatment beyond RECIST-defined progression in recurrent or metastatic squamous cell carcinoma of the head and neck in CheckMate 141: A subgroup analysis of a randomized phase 3 clinical trial. Cancer, 125:3208-3218, 2019
16. Imamura Y, Kiyota N, Ogawa G, Akimoto T, Fujii M, Hanai N, Iwae S, Monden N, Matsuura K, Onozawa Y, Hayashi R, Tahara M. Nutritional support dependence after curative chemoradiotherapy in head and neck cancer: supplementary analysis of a phase II trial (JCOG0706S1). Jpn J Clin Oncol, 49:1009-1015, 2019
