Annual Report 2019
Department of Experimental Therapeutics
Toshihiko Doi, Yasutoshi Kuboki, Kiyotaka Yoh, Yoichi Naito, Kohei Shitara, Takahiro Kogawa, Hideaki Takahashi, Kenichi Harano, Junichiro Yuda, Nobuhiko Yamauchi, Shigehiro Koganemaru
Introduction
The NCC-EPOC Phase I Group was organized to promote the early drug development, especially the first in human (FIH) trial, and in 2012 the phase I group comprised two sub-units (NCCE-Kashiwa & NCC-Tsukiji) organized by each hospital. The goal of both of (or each of) the units was to perform initial clinical evaluation of promising new anti-cancer compounds emerging from the laboratory. Our Phase 1 unit is the largest program in Japan - indeed in Asia - and we help develop new cancer drugs through early-phase trials.
In April 2013, the Department of Experimental Therapeutics was launched to strongly promote the EPOC missions as previously described. The members of the Department of Experimental Therapeutics comprised specialists in oncology fields. We also contributed to IIT using unapproved drugs and new academic seeds.
The Team and What We Do
Our team has conducted and managed early drug development, especially first-in-human (FIH) trials.
Research activities
This department is key to developing new anti-cancer drugs in our center as well as nationally and while conducting FIH trials is the top priority, we also perform phase I trials. Recently, we joined the global phase I trial to accelerate new drug development in Japan. Video or teleconferences have been held with the EU and US sites and we are discussing details of patient enrollment as well as further developmental strategy. Routine web conferences are also held between Kashiwa and Tsukiji campuses every Friday morning and we share information about adverse events and patient enrollment as well as referring candidates to each other to accelerate enrollment. Several IIT-FIH using new class seeds are conducted by each unit and also unapproved company agents.
Clinical trials
In 2019, 42 phase I trials were conducted. (Table 1).
Table 1. Number of pathology and cytology samples examined in the Pathology Division in 2019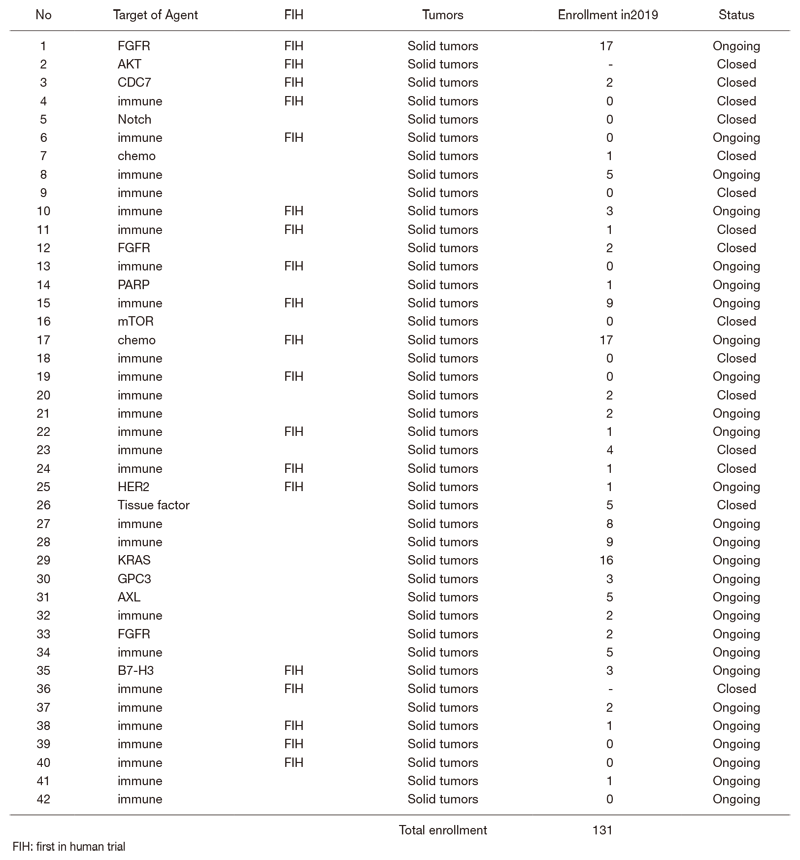

List of papers published in 2019
Journal
1. Moritani K, Kanemitsu Y, Shida D, Shitara K, Mizusawa J, Katayama H, Hamaguchi T, Shimada Y. A randomized controlled trial comparing primary tumour resection plus chemotherapy with chemotherapy alone in incurable stage IV colorectal cancer: JCOG1007 (iPACS study). Jpn J Clin Oncol, 50:89-93, 2020
2. Hatogai K, Fujii S, Kitano S, Kojima T, Daiko H, Yoshino T, Ohtsu A, Takiguchi Y, Doi T, Ochiai A. Relationship between the immune microenvironment of different locations in a primary tumour and clinical outcomes of oesophageal squamous cell carcinoma. Br J Cancer, 122:413-420, 2020
3. Shimokawa M, Nosaki K, Seto T, Ohashi K, Morise M, Horinouchi H, Sakakibara J, Murakami H, Yano S, Satouchi M, Matsumoto S, Goto K, Yoh K. Phase II, open-label, multicenter trial of crizotinib in Japanese patients with advanced non-small cell lung cancer harboring a MET gene alteration: Co-MET study. Trials, 21:298, 2020
4. Kawazoe A, Shitara K. Trifluridine/tipiracil for the treatment of metastatic gastric cancer. Expert Rev Gastroenterol Hepatol, 14:65-70, 2020
5. Shitara K, Hara H, Yoshikawa T, Fujitani K, Nishina T, Hosokawa A, Asakawa T, Kawakami S, Muro K. Pertuzumab plus trastuzumab and chemotherapy for Japanese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subgroup analysis of the JACOB trial. Int J Clin Oncol, 25:301-311, 2020
6. Shitara K, Honma Y, Omuro Y, Yamaguchi K, Chin K, Muro K, Nakagawa S, Kawakami S, Hironaka S, Nishina T. Efficacy of trastuzumab emtansine in Japanese patients with previously treated HER2-positive locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma: A subgroup analysis of the GATSBY study. Asia Pac J Clin Oncol, 16:5-13, 2020
7. Naito Y, Mishima S, Akagi K, Igarashi A, Ikeda M, Okano S, Kato S, Takano T, Tsuchihara K, Terashima K, Nishihara H, Nishiyama H, Hiyama E, Hirasawa A, Hosoi H, Maeda O, Yatabe Y, Okamoto W, Ono S, Kajiyama H, Nagashima F, Hatanaka Y, Miyachi M, Kodera Y, Yoshino T, Taniguchi H. Japan society of clinical oncology/Japanese society of medical oncology-led clinical recommendations on the diagnosis and use of tropomyosin receptor kinase inhibitors in adult and pediatric patients with neurotrophic receptor tyrosine kinase fusion-positive advanced solid tumors, cooperated by the Japanese society of pediatric hematology/oncology. Int J Clin Oncol, 25:403-417, 2020
8. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol, 38:1-10, 2020
9. Demachi K, Bando H, Nomura H, Shitara K, Yoshino T, Yamaguchi M, Kawasaki T. Clinical impact of renal impairment on the safety and efficacy of S-1 plus oxaliplatin in patients with advanced gastric cancer: a single institutional study. Jpn J Clin Oncol, 50:129-137, 2020
10. Kogawa T, Fujii T, Wu J, Harano K, Fouad TM, Liu DD, Shen Y, Masuda H, Krishnamurthy S, Chavez-MacGregor M, Lim B, Murthy RK, Valero V, Tripathy D, Ueno NT. Prognostic Value of HER2 to CEP17 Ratio on Fluorescence In Situ Hybridization Ratio in Patients with Nonmetastatic HER2-Positive Inflammatory and Noninflammatory Breast Cancer Treated with Neoadjuvant Chemotherapy with or without Trastuzumab. Oncologist, 25:e909-e919, 2020
11. Kato H, de Souza P, Kim SW, Lickliter JD, Naito Y, Park K, Kumar S, Mugundu GM, Bang YJ. Safety, Pharmacokinetics, and Clinical Activity of Adavosertib in Combination with Chemotherapy in Asian Patients with Advanced Solid Tumors: Phase Ib Study. Target Oncol, 15:75-84, 2020
12. Naito Y, Kai Y, Ishikawa T, Fujita T, Uehara K, Doihara H, Tokunaga S, Shimokawa M, Ito Y, Saeki T. Chemotherapy-induced nausea and vomiting in patients with breast cancer: a prospective cohort study. Breast Cancer, 27:122-128, 2020
13. Nishida T, Sakai Y, Takagi M, Ozaka M, Kitagawa Y, Kurokawa Y, Masuzawa T, Naito Y, Kagimura T, Hirota S. Adherence to the guidelines and the pathological diagnosis of high-risk gastrointestinal stromal tumors in the real world. Gastric Cancer, 23:118-125, 2020
14. Yuda J, Odawara J, Minami M, Muta T, Kohno K, Tanimoto K, Eto T, Shima T, Kikushige Y, Kato K, Takenaka K, Iwasaki H, Minami Y, Ohkawa Y, Akashi K, Miyamoto T. Tyrosine kinase inhibitors induce alternative spliced BCR-ABL(Ins35bp) variant via inhibition of RNA polymerase II on genomic BCR-ABL. Cancer Sci, 111:2361-2373, 2020
15. Kobayashi E, Naito Y, Asano N, Maejima A, Endo M, Takahashi S, Megumi Y, Kawai A. Interim results of a real-world observational study of eribulin in soft tissue sarcoma including rare subtypes. Jpn J Clin Oncol, 49:938-946, 2019
16. Doi T, Aramaki T, Yasui H, Muro K, Ikeda M, Okusaka T, Inaba Y, Nakai K, Ikezawa H, Nakajima R. A phase I study of ontuxizumab, a humanized monoclonal antibody targeting endosialin, in Japanese patients with solid tumors. Invest New Drugs, 37:1061-1074, 2019
17. Naito T, Umemura S, Nakamura H, Zenke Y, Udagawa H, Kirita K, Matsumoto S, Yoh K, Niho S, Motoi N, Aokage K, Tsuboi M, Ishii G, Goto K. Successful treatment with nivolumab for SMARCA4-deficient non-small cell lung carcinoma with a high tumor mutation burden: A case report. Thorac Cancer, 10:1285-1288, 2019
18. Naito T, Udagawa H, Umemura S, Sakai T, Zenke Y, Kirita K, Matsumoto S, Yoh K, Niho S, Tsuboi M, Ishii G, Goto K. Non-small cell lung cancer with loss of expression of the SWI/SNF complex is associated with aggressive clinicopathological features, PD-L1-positive status, and high tumor mutation burden. Lung Cancer, 138:35-42, 2019
19. Doi T, Fujiwara Y, Matsubara N, Tomomatsu J, Iwasa S, Tanaka A, Endo-Tsukude C, Nakagawa S, Takahashi S. Phase I study of ipatasertib as a single agent and in combination with abiraterone plus prednisolone in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 84:393-404, 2019
20. Doi T, Iwasa S, Muro K, Satoh T, Hironaka S, Esaki T, Nishina T, Hara H, Machida N, Komatsu Y, Shimada Y, Otsu S, Shimizu S, Watanabe M. Phase 1 trial of avelumab (anti-PD-L1) in Japanese patients with advanced solid tumors, including dose expansion in patients with gastric or gastroesophageal junction cancer: the JAVELIN Solid Tumor JPN trial. Gastric Cancer, 22:817-827, 2019
21. Doi T, Muro K, Ishii H, Kato T, Tsushima T, Takenoyama M, Oizumi S, Gemmoto K, Suna H, Enokitani K, Kawakami T, Nishikawa H, Yamamoto N. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin Cancer Res, 25:6614-6622, 2019
22. Naito T, Udagawa H, Sato J, Horinouchi H, Murakami S, Goto Y, Kanda S, Fujiwara Y, Yamamoto N, Zenke Y, Kirita K, Matsumoto S, Yoh K, Niho S, Motoi N, Ohe Y, Ishii G, Goto K. A Minimum Of 100 Tumor Cells in a Single Biopsy Sample Is Required to Assess Programmed Cell Death Ligand 1 Expression in Predicting Patient Response to Nivolumab Treatment in Nonsquamous Non-Small Cell Lung Carcinoma. J Thorac Oncol, 14:1818-1827, 2019
23. Shimomura A, Yonemori K, Yoshida M, Yoshida T, Yasojima H, Masuda N, Aogi K, Takahashi M, Naito Y, Shimizu S, Nakamura R, Hamada A, Michimae H, Hashimoto J, Yamamoto H, Kawachi A, Shimizu C, Fujiwara Y, Tamura K. Gene Alterations in Triple-Negative Breast Cancer Patients in a Phase I/II Study of Eribulin and Olaparib Combination Therapy. Transl Oncol, 12:1386-1394, 2019
24. Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, Tsurutani J, Kadowaki S, Yamaguchi K, Iwasa S, Saito K, Fujisaki Y, Sugihara M, Shahidi J, Doi T. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol, 20:827-836, 2019
25. Shitara K, Satoh T, Iwasa S, Yamaguchi K, Muro K, Komatsu Y, Nishina T, Esaki T, Hasegawa J, Kakurai Y, Kamiyama E, Nakata T, Nakamura K, Sakaki H, Hyodo I. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the afucosylated, humanized anti-EPHA2 antibody DS-8895a: a first-in-human phase I dose escalation and dose expansion study in patients with advanced solid tumors. J Immunother Cancer, 7:219, 2019
26. Yamada Y, Boku N, Mizusawa J, Iwasa S, Kadowaki S, Nakayama N, Azuma M, Sakamoto T, Shitara K, Tamura T, Chin K, Hata H, Nakamori M, Hara H, Yasui H, Katayama H, Fukuda H, Yoshikawa T, Sasako M, Terashima M. Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): an open-label, phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol, 4:501-510, 2019
27. Kenmotsu H, Yoh K, Mori K, Ono A, Baba T, Fujiwara Y, Yamaguchi O, Ko R, Okamoto H, Yamamoto N, Ninomiya T, Ogura T, Kato T. Phase II study of nab-paclitaxel + carboplatin for patients with non-small-cell lung cancer and interstitial lung disease. Cancer Sci, 110:3738-3745, 2019
28. Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L, Chiu CH, Park K, Novello S, Nadal E, Imamura F, Yoh K, Shih JY, Au KH, Moro-Sibilot D, Enatsu S, Zimmermann A, Frimodt-Moller B, Visseren-Grul C, Reck M. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol, 20:1655-1669, 2019
29. Ota T, Kirita K, Matsuzawa R, Udagawa H, Matsumoto S, Yoh K, Niho S, Ishii G, Goto K. Validity of using immunohistochemistry to predict treatment outcome in patients with non-small cell lung cancer not otherwise specified. J Cancer Res Clin Oncol, 145:2495-2506, 2019
30. Doi T, Yoh K, Shitara K, Takahashi H, Ueno M, Kobayashi S, Morimoto M, Okusaka T, Ueno H, Morizane C, Okano N, Nagashima F, Furuse J. First-in-human phase 1 study of novel dUTPase inhibitor TAS-114 in combination with S-1 in Japanese patients with advanced solid tumors. Invest New Drugs, 37:507-518, 2019
31. Ikemura S, Yasuda H, Matsumoto S, Kamada M, Hamamoto J, Masuzawa K, Kobayashi K, Manabe T, Arai D, Nakachi I, Kawada I, Ishioka K, Nakamura M, Namkoong H, Naoki K, Ono F, Araki M, Kanada R, Ma B, Hayashi Y, Mimaki S, Yoh K, Kobayashi SS, Kohno T, Okuno Y, Goto K, Tsuchihara K, Soejima K. Molecular dynamics simulation-guided drug sensitivity prediction for lung cancer with rare EGFR mutations. Proc Natl Acad Sci U S A, 116:10025-10030, 2019
32. Nakamura M, Kageyama SI, Niho S, Okumura M, Hojo H, Motegi A, Nakamura N, Zenda S, Yoh K, Goto K, Akimoto T. Impact of EGFR Mutation and ALK Translocation on Recurrence Pattern After Definitive Chemoradiotherapy for Inoperable Stage III Non-squamous Non-small-cell Lung Cancer. Clin Lung Cancer, 20:e256-e264, 2019
33. Esaki T, Hirai F, Makiyama A, Seto T, Bando H, Naito Y, Yoh K, Ishihara K, Kakizume T, Natsume K, Myers A, Doi T. Phase I dose-escalation study of capmatinib (INC280) in Japanese patients with advanced solid tumors. Cancer Sci, 110:1340-1351, 2019
34. Shitara K, Yodo Y, Iino S. A Phase I Study of Napabucasin Plus Paclitaxel for Japanese Patients With Advanced/Recurrent Gastric Cancer. In Vivo, 33:933-937, 2019
35. Kawazoe A, Shitara K. Next-generation sequencing and biomarkers for gastric cancer: what is the future? Ther Adv Med Oncol, 11:1758835919848189, 2019
36. Sasaki A, Nakamura Y, Mishima S, Kawazoe A, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, Kuwata T, Akimoto T, Shitara K. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer, 22:793-802, 2019
37. Ishii T, Kawazoe A, Shitara K. Dawn of precision medicine on gastric cancer. Int J Clin Oncol, 24:779-788, 2019
38. Shitara K, Ueha S, Shichino S, Aoki H, Ogiwara H, Nakatsura T, Suzuki T, Shimomura M, Yoshikawa T, Shoda K, Kitano S, Yamashita M, Nakayama T, Sato A, Kuroda S, Wakabayashi M, Nomura S, Yokochi S, Ito S, Matsushima K, Doi T. First-in-human phase 1 study of IT1208, a defucosylated humanized anti-CD4 depleting antibody, in patients with advanced solid tumors. J Immunother Cancer., 7:195, 2019
39. Koganemaru S, Kuboki Y, Koga Y, Kojima T, Yamauchi M, Maeda N, Kagari T, Hirotani K, Yasunaga M, Matsumura Y, Doi T. U3-1402, a Novel HER3-Targeting Antibody-Drug Conjugate, for the Treatment of Colorectal Cancer. Mol Cancer Ther, 18:2043-2050, 2019
40. Doi T, Kurokawa Y, Sawaki A, Komatsu Y, Ozaka M, Takahashi T, Naito Y, Ohkubo S, Nishida T. Efficacy and safety of TAS-116, an oral inhibitor of heat shock protein 90, in patients with metastatic or unresectable gastrointestinal stromal tumour refractory to imatinib, sunitinib and regorafenib: a phase II, single-arm trial. Eur J Cancer, 121:29-39, 2019
41. Kotani D, Kuboki Y, Horasawa S, Kaneko A, Nakamura Y, Kawazoe A, Bando H, Taniguchi H, Shitara K, Kojima T, Tsuji A, Yoshino T. Retrospective cohort study of trifluridine/tipiracil (TAS-102) plus bevacizumab versus trifluridine/tipiracil monotherapy for metastatic colorectal cancer. BMC Cancer, 19:1253, 2019
42. Kato K, Shah MA, Enzinger P, Bennouna J, Shen L, Adenis A, Sun JM, Cho BC, Özgüroğlu M, Kojima T, Kostorov V, Hierro C, Zhu Y, McLean LA, Shah S, Doi T. KEYNOTE-590: Phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol, 15:1057-1066, 2019
43. Masuishi T, Taniguchi H, Kotani D, Bando H, Komatsu Y, Shinozaki E, Nakajima TE, Satoh T, Nishina T, Esaki T, Wakabayashi M, Nomura S, Takahashi K, Ono H, Hirano N, Fujishiro N, Fuse N, Sato A, Ohtsu A, Yoshino T. Rationale and design of the BRAVERY study (EPOC1701): a multicentre phase II study of eribulin in patients with BRAF V600E mutant metastatic colorectal cancer. ESMO Open, 4:e000590, 2019
44. Harding JJ, Bauer TM, Tan DSW, Bedard PL, Rodon J, Doi T, Schnell C, Iyer V, Baffert F, Radhakrishnan R, Fabre C, Juric D. Characterization and phase I study of CLR457, an orally bioavailable pan-class I PI3-kinase inhibitor. Invest New Drugs, 37:271-281, 2019
45. Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, Morikawa H, Kawazoe A, Kinoshita T, Shitara K, Sakaguchi S, Nishikawa H. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A, 116:9999-10008, 2019
46. Tamura K, Tsurutani J, Takahashi S, Iwata H, Krop IE, Redfern C, Sagara Y, Doi T, Park H, Murthy RK, Redman RA, Jikoh T, Lee C, Sugihara M, Shahidi J, Yver A, Modi S. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol, 20:816-826, 2019
47. Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol, 16:356-371, 2019
48. Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, Borg C, Doi T, Yoon HH, Savage MJ, Wang J, Dalal RP, Shah S, Wainberg ZA, Chung HC. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer, 22:828-837, 2019
49. Yoshikawa T, Muro K, Shitara K, Oh DY, Kang YK, Chung HC, Kudo T, Chin K, Kadowaki S, Hamamoto Y, Hironaka S, Yoshida K, Yen CJ, Omuro Y, Bai LY, Maeda K, Ozeki A, Yoshikawa R, Kitagawa Y. Effect of First-line S-1 Plus Oxaliplatin With or Without Ramucirumab Followed by Paclitaxel Plus Ramucirumab on Advanced Gastric Cancer in East Asia: The Phase 2 RAINSTORM Randomized Clinical Trial. JAMA Netw Open, 2:e198243, 2019
50. Kaito A, Kuwata T, Tokunaga M, Shitara K, Sato R, Akimoto T, Kinoshita T. HER2 heterogeneity is a poor prognosticator for HER2-positive gastric cancer. World J Clin Cases, 7:1964-1977, 2019
51. Fujii S, Yoshino T, Yamazaki K, Muro K, Yamaguchi K, Nishina T, Yuki S, Shinozaki E, Shitara K, Bando H, Mimaki S, Nakai C, Matsushima K, Suzuki Y, Akagi K, Yamanaka T, Nomura S, Esumi H, Sugiyama M, Nishida N, Mizokami M, Koh Y, Abe Y, Ohtsu A, Tsuchihara K. Histopathological factors affecting the extraction of high quality genomic DNA from tissue sections for next-generation sequencing. Biomed Rep, 11:171-180, 2019
52. Lin CH, Yap YS, Lee KH, Im SA, Naito Y, Yeo W, Ueno T, Kwong A, Li H, Huang SM, Leung R, Han W, Tan B, Hu FC, Huang CS, Cheng AL, Lu YS. Contrasting Epidemiology and Clinicopathology of Female Breast Cancer in Asians vs the US Population. J Natl Cancer Inst, 111:1298-1306, 2019
53. Yeo W, Ueno T, Lin CH, Liu Q, Lee KH, Leung R, Naito Y, Park YH, Im SA, Li H, Yap YS, Lu YS. Treating HR+/HER2- breast cancer in premenopausal Asian women: Asian Breast Cancer Cooperative Group 2019 Consensus and position on ovarian suppression. Breast Cancer Res Treat, 177:549-559, 2019
