Annual Report 2019
Department of Head and Neck Medical Oncology
Narikazu Boku, Yoshitaka Honma
Introduction
The Department of Head and Neck Medical Oncology focuses on the development of new drugs and establishment of standard chemotherapy regimens including multimodality treatment with surgery and/or radiotherapy for advanced head and neck cancers (HNC), consisting of malignancies arising from the oral cavity, nasopharynx, oropharynx, hypopharynx, larynx, nasal/paranasal cavity, salivary gland, ear canal, and thyroid and so on. The main histology of HNC is squamous cell carcinoma (HNSCC), but there is a wide variety of histological types especially in the nasal/paranasal cavity and salivary glands. Therefore, pathological diagnosis is essential, making a treatment strategy based on pathological findings very important in advanced HNC.
The Team and What We Do
The staff of the Department of Head and Neck Medical Oncology consists of 2 medical oncologists. We hold a daily case conference together at 5 pm after finishing routine clinical work and also hold a monthly research conference and discuss the progress of clinical trials or in-house research. Intergroup meetings with the Departments of Head and Neck Surgery, Radiation Oncology, Diagnostic Radiology, and Diagnostic Pathology are held weekly to decide optimal treatment strategies for each individual case and to discuss treatment consensus for the disease. In 2019, we treated 159 hospitalized patients (86 of whom were newly diagnosed). Of these patients, 6 were entered into clinical trials.
Research activities
A trans-oral/percutaneous biopsy and blood sampling before and after cetuximab and nivolumab provide an excellent opportunity to study biomarkers. We are collecting these fresh samples from patients with HNSCC to evaluate the correlations between gene expression or immunogenic profiles and patients’ outcomes by using genome sequencing, immune-panel, microarray, or real-time PCR techniques. We have also been measuring the gene expressions of possible predictive biomarkers by using FFPE samples obtained from surgical resection or trans-oral/percutaneous biopsy. These studies are being performed in collaboration with the Center for Medical Genomics, National Cancer Center Research Institute, or other institutions.
Clinical trials (Table 1)
We carried out several clinical trials in collaboration with the Departments of Head and Neck Surgery, Radiation Oncology, Diagnostic Radiology, and Diagnostic Pathology in our hospital or other institutions. Details of clinical trials are summarized in the Table, including JCOG (Japan Clinical Oncology Group) trials, company-initiated trials, and other collaborative investigator-initiated trials.
1. Palliative chemotherapy for recurrent/metastatic HNSCC (RM-HNSCC)
For 1st line treatment, the pembro-based regimen has been a new standard regimen for HNSCC. A phase III trial comparing Nivo plus ipilimumab with the EXTREME regimen (CheckMate-651) as 1st-line treatment for metastatic HNSCC has finished patient accrual. For 2nd line treatment, a randomized phase III trial comparing photoimmunotherapy (ASP-1929) and a taxane for HNSCC with recurrence only in the locoregional area are also ongoing.
Table 1. Clinical trial for new drug development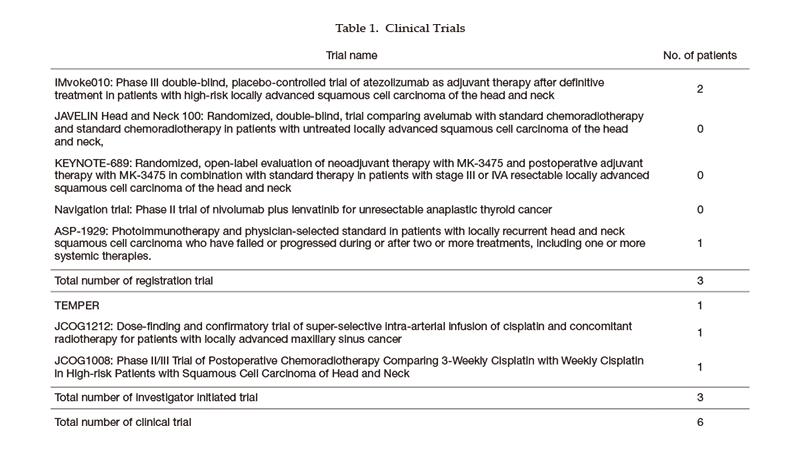

2. Multimodality treatment for locally advanced HNSCC (LA-HNSCC)
A phase III trial investigating the additive effect of avelumab (anti-PD-L1 antibody) on definitive CDDP+RT for locally advanced HNSCC was reported as a negative trial based on the results of interim analysis. The IMvoke010 trial investigating the additive effect of atezolizumab (anti-PD-L1 antibody) as maintenance therapy after multidisciplinary treatment has finished patient accrual.
The KEYNOTE-689 trial investigating the additive effect of perioperative pembro on surgery plus adjuvant treatment for locally advanced HNSCC is also ongoing.
3. Palliative chemotherapy for recurrent/metastatic thyroid cancer
For anaplastic thyroid cancer, no standard treatment has been established. However, lenvatinib showed moderate antitumor activity even for anaplastic thyroid cancer in a phase II trial, so it was made available for anaplastic thyroid cancer in clinical practice. An investigator-initiated phase I/II trial evaluating the efficacy and feasibility of lenvatinib plus Nivo is ongoing.
Education
We performed on-the-job training with the residents every day. Each member of staff gives a lecture for the residents every 3 months. Each resident is given a research theme, and proceeds with it with the staff. After analysis of the data, the residents are given the opportunity of presenting at medical congresses and creating a manuscript for medical journals.
Future prospects
In April 2020, the chief of the Department of Head and Neck Medical Oncology changed, from Dr. Boku to Dr. Kato. Also, esophageal cancer has become one of the focuses of our department. In May 2020, Dr. Yamamoto newly joined the staff of our department. From 2020, our mission is to develop new drugs and strategies with a multi-modal approach for not only head and neck cancer but also esophageal cancer. We have conducted an investigator-initiated trial with nivolumab for patients with resectable esophageal cancer. In this trial, we also conducted translational research using biopsy or blood samples to predict the efficacy of nivolumab. We plan to host a web conference with foreign investigators in Taiwan and Korea using a web conference system. This may be a good opportunity for young investigators to give a presentation in English.
List of papers published in 2019
Journal
1. Yamamoto Y, Kondo S, Matsuzaki J, Esaki M, Okusaka T, Shimada K, Murakami Y, Enomoto M, Tamori A, Kato K, Aoki Y, Takizawa S, Sakamoto H, Niida S, Takeshita F, Ochiya T. Highly Sensitive Circulating MicroRNA Panel for Accurate Detection of Hepatocellular Carcinoma in Patients With Liver Disease. Hepatol Commun, 4:284-297, 2020
2. Honma Y, Nagashima K, Hirano H, Shoji H, Iwasa S, Takashima A, Okita N, Kato K, Boku N, Murakami N, Inaba K, Ito Y, Itami J, Kanamori J, Oguma J, Daiko H. Clinical outcomes of locally advanced esophageal neuroendocrine carcinoma treated with chemoradiotherapy. Cancer Med, 9:595-604, 2020
3. Kadota T, Abe S, Yoda Y, Yoshinaga S, Oda I, Kojima T, Kato K, Daiko H, Yano T. Clinical outcomes according to the modified endoscopic criteria for neoadjuvant chemotherapy in resectable esophageal squamous cell carcinoma. Dig Endosc, 32:337-345, 2020
4. Satomi-Tsushita N, Honma Y, Nagashima K, Ito Y, Hirano H, Shoji H, Takashima A, Iwasa S, Kato K, Hamaguchi T, Itami J, Boku N. Risk Factors of Severe Benign Cicatricial Stricture After Definitive Chemoradiation for Localized T3 Esophageal Carcinoma. Anticancer Res, 40:1071-1077, 2020
5. Sudo K, Kato K, Matsuzaki J, Takizawa S, Aoki Y, Shoji H, Iwasa S, Honma Y, Takashima A, Sakamoto H, Naka T, Sekine S, Boku N, Ochiya T. Identification of serum microRNAs predicting the response of esophageal squamous-cell carcinoma to nivolumab. Jpn J Clin Oncol, 50:114-121, 2020
6. Murakami N, Mori T, Kubo Y, Yoshimoto S, Ito K, Honma Y, Ueno T, Kobayashi K, Okamoto H, Boku N, Takahashi K, Inaba K, Okuma K, Igaki H, Nakayama Y, Itami J. Prognostic impact of immunohistopathologic features in definitive radiation therapy for nasopharyngeal cancer patients. J Radiat Res, 61:161-168, 2020
7. Asakura K, Kadota T, Matsuzaki J, Yoshida Y, Yamamoto Y, Nakagawa K, Takizawa S, Aoki Y, Nakamura E, Miura J, Sakamoto H, Kato K, Watanabe SI, Ochiya T. A miRNA-based diagnostic model predicts resectable lung cancer in humans with high accuracy. Commun Biol, 3:134, 2020
8. Shitara K, Honma Y, Omuro Y, Yamaguchi K, Chin K, Muro K, Nakagawa S, Kawakami S, Hironaka S, Nishina T. Efficacy of trastuzumab emtansine in Japanese patients with previously treated HER2-positive locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma: A subgroup analysis of the GATSBY study. Asia Pac J Clin Oncol, 16:5-13, 2020
9. Yokota T, Kato K, Hamamoto Y, Tsubosa Y, Ogawa H, Ito Y, Hara H, Ura T, Kojima T, Chin K, Hironaka S, Kii T, Kojima Y, Akutsu Y, Matsushita H, Kawakami K, Mori K, Makiuchi T, Nagumo R, Kitagawa Y. A 3-Year Overall Survival Update From a Phase 2 Study of Chemoselection With DCF and Subsequent Conversion Surgery for Locally Advanced Unresectable Esophageal Cancer. Ann Surg Oncol, 27:460-467, 2020
10. Sunami K, Ichikawa H, Kubo T, Kato M, Fujiwara Y, Shimomura A, Koyama T, Kakishima H, Kitami M, Matsushita H, Furukawa E, Narushima D, Nagai M, Taniguchi H, Motoi N, Sekine S, Maeshima A, Mori T, Watanabe R, Yoshida M, Yoshida A, Yoshida H, Satomi K, Sukeda A, Hashimoto T, Shimizu T, Iwasa S, Yonemori K, Kato K, Morizane C, Ogawa C, Tanabe N, Sugano K, Hiraoka N, Tamura K, Yoshida T, Fujiwara Y, Ochiai A, Yamamoto N, Kohno T. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci, 110:1480-1490, 2019
11. Sudo K, Kato K, Matsuzaki J, Boku N, Abe S, Saito Y, Daiko H, Takizawa S, Aoki Y, Sakamoto H, Niida S, Takeshita F, Fukuda T, Ochiya T. Development and Validation of an Esophageal Squamous Cell Carcinoma Detection Model by Large-Scale MicroRNA Profiling. JAMA Netw Open, 2:e194573, 2019
12. Terada M, Hara H, Daiko H, Mizusawa J, Kadota T, Hori K, Ogawa H, Ogata T, Sakanaka K, Sakamoto T, Kato K, Kitagawa Y. Phase III study of tri-modality combination therapy with induction docetaxel plus cisplatin and 5-fluorouracil versus definitive chemoradiotherapy for locally advanced unresectable squamous-cell carcinoma of the thoracic esophagus (JCOG1510: TRIANgLE). Jpn J Clin Oncol, 49:1055-1060, 2019
13. Ito T, Honma Y, Hirano H, Shoji H, Okita N, Iwasa S, Takashima A, Kato K, Boku N. S-1 Monotherapy After Failure of Platinum Plus 5-Fluorouracil Chemotherapy in Recurrent or Metastatic Esophageal Carcinoma. Anticancer Res, 39:3931-3936, 2019
14. Monma S, Kato K, Shouji H, Okita N, Takashima A, Honma Y, Iwasa S, Hamaguchi T, Yamada Y, Shimada Y, Boku N, Nagashima K, Ito Y, Itami J. Gastric mucosal injury and hemorrhage after definitive chemoradiotherapy for locally advanced esophageal cancer. Esophagus, 16:402-407, 2019
15. Murakami N, Mori T, Nakamura S, Yoshimoto S, Honma Y, Ueno T, Kobayashi K, Kashihara T, Takahashi K, Inaba K, Okuma K, Igaki H, Nakayama Y, Itami J. Prognostic value of the expression of epithelial cell adhesion molecules in head and neck squamous cell carcinoma treated by definitive radiotherapy. J Radiat Res, 60:803-811, 2019
16. Nakano MH, Udagawa C, Shimo A, Kojima Y, Yoshie R, Zaha H, Abe N, Motonari T, Unesoko M, Tamura K, Shimoi T, Yoshida M, Yoshida T, Sakamoto H, Kato K, Mushiroda T, Tsugawa K, Zembutsu H. A Genome-Wide Association Study Identifies Five Novel Genetic Markers for Trastuzumab-Induced Cardiotoxicity in Japanese Population. Biol Pharm Bull, 42:2045-2053, 2019
17. Hirano H, Kato K. Systemic treatment of advanced esophageal squamous cell carcinoma: chemotherapy, molecular-targeting therapy and immunotherapy. Jpn J Clin Oncol, 49:412-420, 2019
18. Satomi-Tsushita N, Shimomura A, Matsuzaki J, Yamamoto Y, Kawauchi J, Takizawa S, Aoki Y, Sakamoto H, Kato K, Shimizu C, Ochiya T, Tamura K. Serum microRNA-based prediction of responsiveness to eribulin in metastatic breast cancer. PLoS One, 14:e0222024, 2019
19. Zhao H, Koyanagi K, Kato K, Ito Y, Itami J, Igaki H, Tachimori Y. Comparison of long-term outcomes between radical esophagectomy and definitive chemoradiotherapy in patients with clinical T1bN0M0 esophageal squamous cell carcinoma. J Thorac Dis, 11:4654-4662, 2019
20. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol, 20:1506-1517, 2019
21. Ohno M, Matsuzaki J, Kawauchi J, Aoki Y, Miura J, Takizawa S, Kato K, Sakamoto H, Matsushita Y, Takahashi M, Miyakita Y, Ichimura K, Narita Y, Ochiya T. Assessment of the Diagnostic Utility of Serum MicroRNA Classification in Patients With Diffuse Glioma. JAMA Netw Open, 2:e1916953, 2019
22. Yamazawa E, Honma Y, Satomi K, Taniguchi H, Takahashi M, Yoshida A, Tominaga K, Miyakita Y, Ohno M, Asanome T, Satomi N, Narita Y. A rare case of brain metastasis from poorly differentiated small bowel adenocarcinoma. Surg Neurol Int, 10:256, 2019
23. Nambu M, Masuda T, Ito S, Kato K, Kojima T, Daiko H, Ito Y, Honda K, Ohtsuki S. Leucine-Rich Alpha-2-Glycoprotein 1 in Serum Is a Possible Biomarker to Predict Response to Preoperative Chemoradiotherapy for Esophageal Cancer. Biol Pharm Bull, 42:1766-1771, 2019
