Annual Report 2019
Department of Breast and Medical Oncology
Kan Yonemori, Tatsunori Shimoi, Emi Noguchi, Akihiko Shimomura, Kazuki Sudo, Maki Tanioka, Hitomi Okuma, Tadaaki Nishikawa, Yaohei Ohtake, Yuki Kojima, Shu Yazaki, Toshihiro Okuya, Yasuhiro Fujiwara and Kenji Tamura
Introduction
The Department of Breast and Medical Oncology provides the most effective treatments by the use of chemotherapy, and works on the establishment of new standards of care for adult malignancies including breast cancer, gynecologic cancer, soft-tissue sarcoma, extragonadal germ cell tumor, cancer of unknown primary and other rare types of solid tumors.
We envision becoming a leading medical oncology department, which makes a difference in cancer care in Japan and in the World. Our mission is to provide patient-centered, state-of-the-art medical care to cancer patients, to develop new effective cancer treatments through clinical and translational research, and to nurture medical oncologists. An evidence-based, research-oriented and multidisciplinary approach is the core value of our practice.
The Team and What We Do
1. Setup
Our division consists of eight full-time attending physicians, two chief residents (fellows), and two to three clinical residents. We also provide educational opportunities to short-term (half-year) residents. Full-time attending physicians are on duty at the outpatient clinic one to two days per week. Inpatient management is undertaken by clinical teams, which consist of attending physicians and residents. A ‘Grand Round’ is scheduled every Monday.
2. Performance
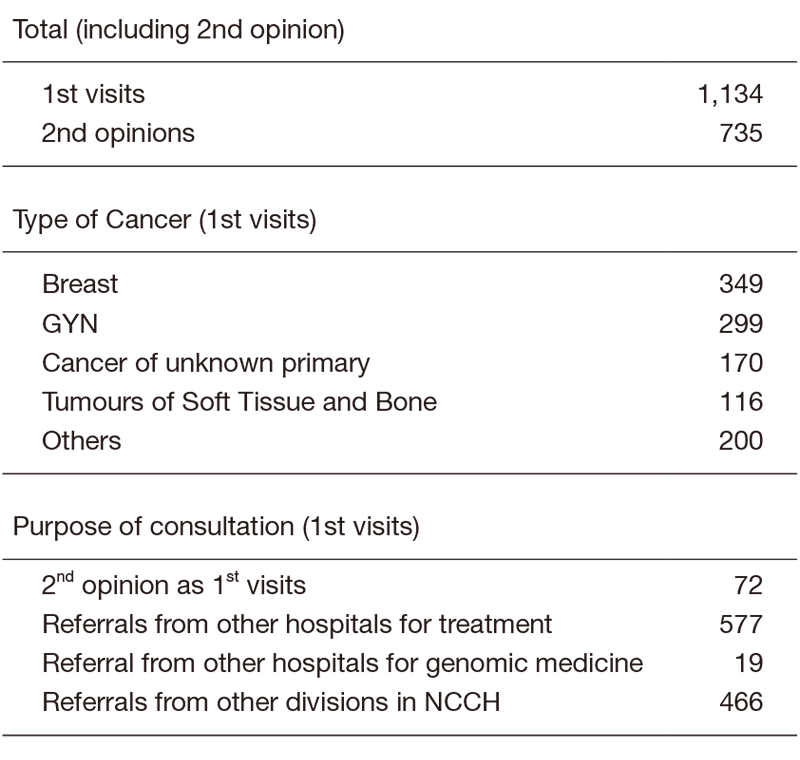
There were 1134 first visits of new patients in 2019 (Table 1). 31% of new patients were breast cancer patients, 26% were gynecological, 15% were primary unknown and 10% were soft-tissue sarcoma in the first visits. We did 735 second opinions in 2019.
3. Conferences
The one-hour briefing medical conferences are held every morning to discuss the evidenced-based care for individual patients. The Phase 1 conference is held on Monday, Journal Club on Wednesday, Clinical Trial conference on Thursday, and the Weekend and Outpatient Follow-up conference on Friday. Multidisciplinary Case Conferences
with diagnostic radiologists, surgeons, and pathologists are held with members of the departments of Breast Surgery, Gynecology, Musculoskeletal Oncology and Rehabilitation, Radiation Oncology and Pathology, each once or twice (Breast) per week, respectively.
The Monthly Breast Cancer Conference is held with the participation of the multidisciplinary specialists to discuss recent topics in breast oncology and to update institutional treatment guidelines. This year, we published “Nyugan-shinnryou Application Notebook” from Nankodo based on these guidelines, which reflects the consensus of the breast team on the body of evidence on breast cancer management.
Research activities
Our research interest extends across a wide range of topics related to treatment and clinical program development. Many of our research programs are secured by public and consignment research grants. In 2019, we conducted many research programs as a primary investigator and participated in additional programs as a co-investigator in research programs secured by competitive public research funds. We published 43 international manuscripts, focusing early-phase anti-cancer drug development, molecular imaging, translational research, novel chemotherapy against sarcoma and ovarian cancer, novel biomarkers to predict efficacy and adverse events of anti-cancer drugs and other basic research. We value cancer survivorship as a research theme in order to develop a comprehensive patient-centered care program.
Clinical trials
In 2019, we actively enrolled patients in phase I studies (including first in human or global) as well as domestic and international phase II and III studies (Table 2). Of note we enrolled patients in IIT for rare cancers. For example, we enrolled them in DS8201, monotherapy in carcinosarcoma patients with HER2 positive in rare tumor patients of all types, as an investigator-initiated clinical trial (IIT in Table 2). We also conducted many types of translational studies (TR) to find novel biomarkers.
Table 2. Active Clinical Trials (2019)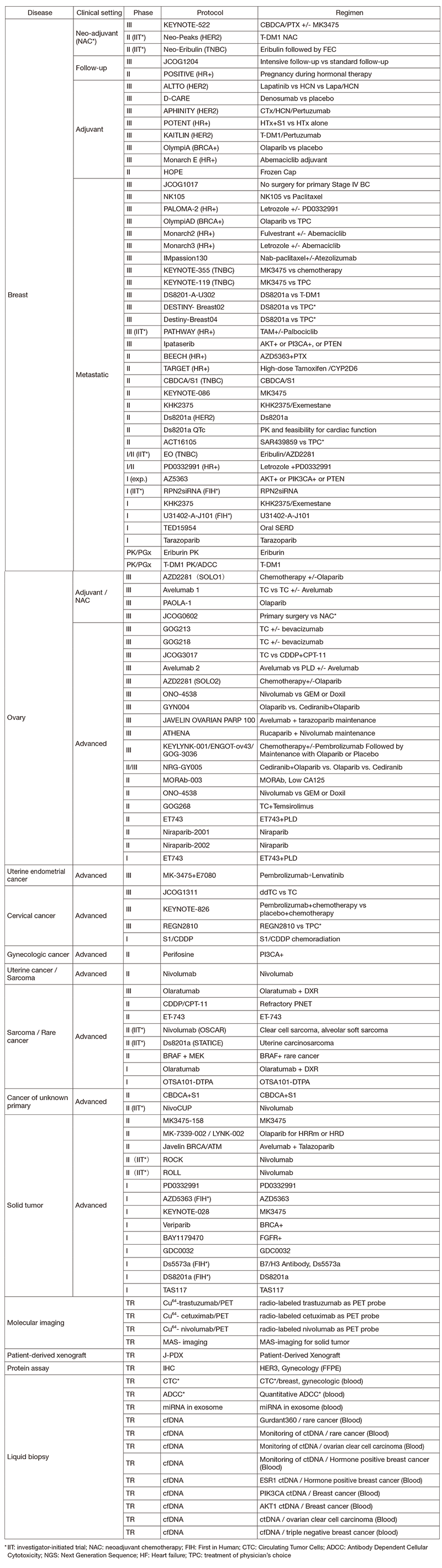

Education
We provide rich educational opportunities to both residents and chief residents through clinical experience as well as research activities. Residents are encouraged to make presentations at local and national conferences. We vigorously support basic, clinical, or translational research conducted by postdoctoral researchers.
Future prospects
We will continue to establish new standard treatments and propose a near-future model of clinical management of adult solid tumors, including breast cancer, and gynecologic cancer. Moreover, we aim to build a comprehensive program, which includes tumor registry, translational research, clinical trials and patient care in rare adult tumors based on our rich clinical experience. We would also like to improve the efficiency of anti-cancer drug development by coordinating basic and translational research in early-phase clinical trials.
List of papers published in 2019
Journal
1. Yoshida A, Arai Y, Hama N, Chikuta H, Bando Y, Nakano S, Kobayashi E, Shibahara J, Fukuhara H, Komiyama M, Watanabe SI, Tamura K, Kawai A, Shibata T. Expanding the clinicopathologic and molecular spectrum of BCOR-associated sarcomas in adults. Histopathology, 76:509-520, 2020
2. Frezza AM, Assi T, Lo Vullo S, Ben-Ami E, Dufresne A, Yonemori K, Noguchi E, Siontis B, Ferraro R, Teterycz P, Duffaud F, Ravi V, Vincenzi B, Gelderblom H, Pantaleo MA, Baldi GG, Desar I, Fedenko A, Maki RG, Jones RL, Benjamin RS, Blay JY, Kawai A, Gounder M, Gronchi A, Le Cesne A, Mir O, Czarnecka AM, Schuetze S, Wagner AJ, Adam J, Barisella M, Sbaraglia M, Hornick JL, Meurgey A, Mariani L, Casali PG, Thornton K, Stacchiotti S. Systemic treatments in MDM2 positive intimal sarcoma: A multicentre experience with anthracycline, gemcitabine, and pazopanib within the World Sarcoma Network. Cancer, 126:98-104, 2020
3. Kato MK, Yunokawa M, Bun S, Shimoi T, Yonemori K, Miyasaka N, Kato T, Tamura K. Treatment strategies for recurrent ovarian cancer in older adult patients in Japan: a study based on real-world data. J Cancer Res Clin Oncol, 146:1335-1341, 2020
4. Kawachi A, Yamashita S, Okochi-Takada E, Hirakawa A, Tsuda H, Shimomura A, Kojima Y, Yonemori K, Fujiwara Y, Kinoshita T, Ushijima T, Tamura K. BRCA1 promoter methylation in breast cancer patients is associated with response to olaparib/eribulin combination therapy. Breast Cancer Res Treat, 181:323-329, 2020
5. Okuma HS, Yonemori K, Narita SN, Sukigara T, Hirakawa A, Shimizu T, Shibata T, Kawai A, Yamamoto N, Nakamura K, Nishida T, Fujiwara Y. MASTER KEY Project: Powering Clinical Development for Rare Cancers Through a Platform Trial. Clin Pharmacol Ther, 2020
6. Seo T, Noguchi E, Yoshida M, Mori T, Tanioka M, Sudo K, Shimomura A, Yonemori K, Fujiwara Y, Tamura K. Response to Dabrafenib and Trametinib of a Patient with Metaplastic Breast Carcinoma Harboring a BRAF V600E Mutation. Case Rep Oncol Med, 2020:2518383, 2020
7. Sudo K, Kato K, Matsuzaki J, Takizawa S, Aoki Y, Shoji H, Iwasa S, Honma Y, Takashima A, Sakamoto H, Naka T, Sekine S, Boku N, Ochiya T. Identification of serum microRNAs predicting the response of esophageal squamous-cell carcinoma to nivolumab. Jpn J Clin Oncol, 50:114-121, 2020
8. Tamura K, Imamura CK, Takano T, Saji S, Yamanaka T, Yonemori K, Takahashi M, Tsurutani J, Nishimura R, Sato K, Kitani A, Ueno NT, Mushiroda T, Kubo M, Fujiwara Y, Tanigawara Y. CYP2D6 Genotype-Guided Tamoxifen Dosing in Hormone Receptor-Positive Metastatic Breast Cancer (TARGET-1): A Randomized, Open-Label, Phase II Study. J Clin Oncol, 38:558-566, 2020
9. Tanabe Y, Shiraishi S, Hashimoto K, Ikeda K, Nishizawa D, Hasegawa J, Shimomura A, Ozaki Y, Tamura N, Yunokawa M, Yonemori K, Takano T, Kawabata H, Tamura K, Fujiwara Y, Shimizu C. Taxane-induced sensory peripheral neuropathy is associated with an SCN9A single nucleotide polymorphism in Japanese patients. BMC Cancer, 20:325, 2020
10. Yazaki S, Yamauchi T, Higashi T. High hepatitis B virus screening rate among patients receiving systemic anticancer treatment in Japan. Int J Clin Oncol, 25:1327-1333, 2020
11. Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, Sohn J, Denduluri N, Perrin C, Aogi K, Tokunaga E, Im SA, Lee KS, Hurvitz SA, Cortes J, Lee C, Chen S, Zhang L, Shahidi J, Yver A, Krop I. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med, 382:610-621, 2020
12. Noda-Narita S, Shimomura A, Tanabe Y, Kawauchi J, Matsuzaki J, Takizawa S, Aoki Y, Shimizu C, Tamura K, Ochiya T. Peripheral neuropathy from paclitaxel: risk prediction by serum microRNAs. BMJ Support Palliat Care, 2020
13. Yazaki S, Hashimoto J, Yamauchi T. Lower response to trastuzumab emtansine in metastatic breast cancer patients with human epidermal growth factor receptor 2 immunohistochemistry score of 2 and fluorescence in situ hybridization positive compared with immunohistochemistry score of 3: a retrospective study. Anti-Cancer Drugs, 2020
14. Shimoi T, Sagara Y, Hara F, Toyama T, Iwata H. First-line endocrine therapy for postmenopausal patients with hormone receptor-positive, HER2-negative metastatic breast cancer: a systematic review and meta-analysis. Breast Cancer, 27:340-346, 2020
15. Turnbull AK, Selli C, Martinez-Perez C, Fernando A, Renshaw L, Keys J, Figueroa JD, He X, Tanioka M, Munro AF, Murphy L, Fawkes A, Clark R, Coutts A, Perou CM, Carey LA, Dixon JM, Sims AH. Unlocking the transcriptomic potential of formalin-fixed paraffin embedded clinical tissues: comparison of gene expression profiling approaches. BMC Bioinformatics, 21:30, 2020
16. Watanabe T, Honda T, Totsuka H, Yoshida M, Tanioka M, Shiraishi K, Shimada Y, Arai E, Ushiama M, Tamura K, Yoshida T, Kanai Y, Kohno T. Simple prediction model for homologous recombination deficiency in breast cancers in adolescents and young adults. Breast Cancer Res Treat, 182:491-502, 2020
17. Sunami K, Ichikawa H, Kubo T, Kato M, Fujiwara Y, Shimomura A, Koyama T, Kakishima H, Kitami M, Matsushita H, Furukawa E, Narushima D, Nagai M, Taniguchi H, Motoi N, Sekine S, Maeshima A, Mori T, Watanabe R, Yoshida M, Yoshida A, Yoshida H, Satomi K, Sukeda A, Hashimoto T, Shimizu T, Iwasa S, Yonemori K, Kato K, Morizane C, Ogawa C, Tanabe N, Sugano K, Hiraoka N, Tamura K, Yoshida T, Fujiwara Y, Ochiai A, Yamamoto N, Kohno T. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci, 110:1480-1490, 2019
18. Sudo K, Kato K, Matsuzaki J, Boku N, Abe S, Saito Y, Daiko H, Takizawa S, Aoki Y, Sakamoto H, Niida S, Takeshita F, Fukuda T, Ochiya T. Development and Validation of an Esophageal Squamous Cell Carcinoma Detection Model by Large-Scale MicroRNA Profiling. JAMA Netw Open, 2:e194573, 2019
19. Goto K, Fujiwara Y, Isobe T, Chayahara N, Kiyota N, Mukohara T, Tsubata Y, Hotta T, Tamura K, Yamamoto N, Minami H. Pharmacokinetic study of the oral fluorouracil antitumor agent S-1 in patients with impaired renal function. Cancer Sci, 110:1987-1994, 2019
20. Hirakawa A, Sudo K, Yonemori K, Sadachi R, Kinoshita F, Kobayashi Y, Okuma HS, Kawachi A, Tamura K, Fujiwara Y, Rubinstein L, Takebe N. A Comparative Study of Longitudinal Toxicities of Cytotoxic Drugs, Molecularly Targeted Agents, Immunomodulatory Drugs, and Cancer Vaccines. Clin Pharmacol Ther, 106:803-809, 2019
21. Inagaki C, Shimoi T, Sumiyoshi Okuma H, Kawachi A, Sudo K, Shimomura A, Noguchi E, Kodaira M, Yunokawa M, Yonemori K, Shimizu C, Arakawa A, Ogawa C, Yoshida A, Fujiwara Y, Tamura K. Bone marrow examination in patients with Ewing sarcoma/peripheral primitive neuroectodermal tumor without metastasis based on (18)F-fluorodeoxyglucose positron emission tomography/computed tomography. Med Oncol, 36:58, 2019
22. Li CK, Dalvi R, Yonemori K, Ariffin H, Lyu CJ, Farid M, Gonzales-Santos JRN, Zhou Q, Bielack S, Brugieres L, Blondeel A, Essiaf S, Peccatori FA, Jezdic S, Stark DP, Douillard JY, Saloustros E, Mountzios G. Care of adolescents and young adults with cancer in Asia: results of an ESMO/SIOPE/SIOP Asia survey. ESMO Open, 4:e000467, 2019
23. Noda-Narita S, Shimomura A, Kawachi A, Sumiyoshi-Okuma H, Sudo K, Shimoi T, Noguchi E, Yonemori K, Shimizu C, Fujiwara Y, Tamura K. Comparison of the efficacy of trastuzumab emtansine between patients with metastatic human epidermal growth factor receptor 2-positive breast cancers previously treated with combination trastuzumab and pertuzumab and with trastuzumab only in Japanese population. Breast Cancer, 26:492-498, 2019
24. Sato J, Shimoi T, Shimomura A, Noguchi E, Kodaira M, Yunokawa M, Yonemori K, Shimizu C, Fujiwara Y, Yoshida M, Tamura K. The Incidence of Nonmalignant Diseases among Patients with Suspected Carcinoma of Unknown Primary Site. Intern Med, 58:1423-1428, 2019
25. Sato J, Shimomura A, Kawauchi J, Matsuzaki J, Yamamoto Y, Takizawa S, Sakamoto H, Ohno M, Narita Y, Ochiya T, Tamura K. Brain metastasis-related microRNAs in patients with advanced breast cancer. PLoS One, 14:e0221538, 2019
26. Shimomura A, Yonemori K, Yoshida M, Yoshida T, Yasojima H, Masuda N, Aogi K, Takahashi M, Naito Y, Shimizu S, Nakamura R, Hamada A, Michimae H, Hashimoto J, Yamamoto H, Kawachi A, Shimizu C, Fujiwara Y, Tamura K. Gene Alterations in Triple-Negative Breast Cancer Patients in a Phase I/II Study of Eribulin and Olaparib Combination Therapy. Transl Oncol, 12:1386-1394, 2019
27. Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, Tsurutani J, Kadowaki S, Yamaguchi K, Iwasa S, Saito K, Fujisaki Y, Sugihara M, Shahidi J, Doi T. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol, 20:827-836, 2019
28. Toki S, Kobayashi E, Yoshida A, Ogura K, Wakai S, Yoshimoto S, Yonemori K, Kawai A. A clinical comparison between dedifferentiated low-grade osteosarcoma and conventional osteosarcoma. Bone Joint J, 101-B:745-752, 2019
29. Yonemori K, Shimomura A, Yasojima H, Masuda N, Aogi K, Takahashi M, Naito Y, Shimizu S, Nakamura R, Hashimoto J, Yamamoto H, Hirakawa A, Michimae H, Hamada A, Yoshida T, Sukigara T, Tamura K, Fujiwara Y. A phase I/II trial of olaparib tablet in combination with eribulin in Japanese patients with advanced or metastatic triple-negative breast cancer previously treated with anthracyclines and taxanes. Eur J Cancer, 109:84-91, 2019
30. Yunokawa M, Sasada S, Takehara Y, Takahashi K, Shimoi T, Yonemori K, Ishikawa M, Kato T, Tamura K. Real-world data on initial treatment strategies for older adult patients with endometrial cancer in Japan. Cancer Chemother Pharmacol, 84:1051-1058, 2019s
31. Kuroda T, Ogiwara H, Sasaki M, Takahashi K, Yoshida H, Kiyokawa T, Sudo K, Tamura K, Kato T, Okamoto A, Kohno T. Therapeutic preferability of gemcitabine for ARID1A-deficient ovarian clear cell carcinoma. Gynecol Oncol, 155:489-498, 2019
32. Bun S, Yunokawa M, Ebata T, Kobayashi Kato M, Shimoi T, Kato T, Tamura K. Feasibility of initial treatment in elderly patients with ovarian cancer in Japan: a retrospective study. Int J Clin Oncol, 24:1111-1118, 2019
33. Kobayashi-Kato M, Yunokawa M, Bun S, Miyasaka N, Kato T, Tamura K. Platinum-free interval affects efficacy of following treatment for platinum-refractory or -resistant ovarian cancer. Cancer Chemother Pharmacol, 84:33-39, 2019
34. Tamura K, Tsurutani J, Takahashi S, Iwata H, Krop IE, Redfern C, Sagara Y, Doi T, Park H, Murthy RK, Redman RA, Jikoh T, Lee C, Sugihara M, Shahidi J, Yver A, Modi S. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol, 20:816-826, 2019
35. Tamura K, Hasegawa K, Katsumata N, Matsumoto K, Mukai H, Takahashi S, Nomura H, Minami H. Efficacy and safety of nivolumab in Japanese patients with uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma: Multicenter, open-label phase 2 trial. Cancer Sci, 110:2894-2904, 2019
36. Nakano MH, Udagawa C, Shimo A, Kojima Y, Yoshie R, Zaha H, Abe N, Motonari T, Unesoko M, Tamura K, Shimoi T, Yoshida M, Yoshida T, Sakamoto H, Kato K, Mushiroda T, Tsugawa K, Zembutsu H. A Genome-Wide Association Study Identifies Five Novel Genetic Markers for Trastuzumab-Induced Cardiotoxicity in Japanese Population. Biol Pharm Bull, 42:2045-2053, 2019
37. Satomi-Tsushita N, Shimomura A, Matsuzaki J, Yamamoto Y, Kawauchi J, Takizawa S, Aoki Y, Sakamoto H, Kato K, Shimizu C, Ochiya T, Tamura K. Serum microRNA-based prediction of responsiveness to eribulin in metastatic breast cancer. PLoS One, 14:e0222024, 2019
38. Hironaka-Mitsuhashi A, Tsuda H, Yoshida M, Shimizu C, Asaga S, Hojo T, Tamura K, Kinoshita T, Ushijima T, Hiraoka N, Fujiwara Y. Invasive breast cancers in adolescent and young adult women show more aggressive immunohistochemical and clinical features than those in women aged 40-44 years. Breast Cancer, 26:386-396, 2019
39. Kubo M, Kawai M, Kumamaru H, Miyata H, Tamura K, Yoshida M, Ogo E, Nagahashi M, Asaga S, Kojima Y, Kadoya T, Aogi K, Niikura N, Miyashita M, Iijima K, Hayashi N, Yamamoto Y, Imoto S, Jinno H. A population-based recurrence risk management study of patients with pT1 node-negative HER2+ breast cancer: a National Clinical Database study. Breast Cancer Res Treat, 178:647-656, 2019
40. Shiino S, Matsuzaki J, Shimomura A, Kawauchi J, Takizawa S, Sakamoto H, Aoki Y, Yoshida M, Tamura K, Kato K, Kinoshita T, Kitagawa Y, Ochiya T. Serum miRNA-based Prediction of Axillary Lymph Node Metastasis in Breast Cancer. Clin Cancer Res, 25:1817-1827, 2019
41. Yuda S, Shimizu C, Yoshida M, Shiino S, Kinoshita T, Maeshima AM, Tamura K. Biomarker discordance between primary breast cancer and bone or bone marrow metastases. Jpn J Clin Oncol, 49:426-430, 2019
42. Takahashi M, Miki S, Fujimoto K, Fukuoka K, Matsushita Y, Maida Y, Yasukawa M, Hayashi M, Shinkyo R, Kikuchi K, Mukasa A, Nishikawa R, Tamura K, Narita Y, Hamada A, Masutomi K, Ichimura K. Eribulin penetrates brain tumor tissue and prolongs survival of mice harboring intracerebral glioblastoma xenografts. Cancer Sci, 110:2247-2257, 2019
43. Udagawa C, Horinouchi H, Shiraishi K, Kohno T, Okusaka T, Ueno H, Tamura K, Ohe Y, Zembutsu H. Whole genome sequencing to identify predictive markers for the risk of drug-induced interstitial lung disease. PLoS One, 14:e0223371, 2019
44. Mehnert JM, Varga A, Brose MS, Aggarwal RR, Lin CC, Prawira A, de Braud F, Tamura K, Doi T, Piha-Paul SA, Gilbert J, Saraf S, Thanigaimani P, Cheng JD, Keam B. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer, 19:196, 2019
45. Noguchi E, Tamura K, Hattori M, Horiguchi J, Sato N, Kanatani K, Matsunaga K, Iwata H, Fujiwara Y. Trastuzumab emtansine plus pertuzumab in Japanese patients with HER2-positive metastatic breast cancer: a phase Ib study. Breast Cancer, 26:39-46, 2019
46. Tanaka R, Yonemori K, Hirakawa A, Kinoshita F, Kobayashi Y, Yamazaki N, Fujimoto M, Tamura K, Fujiwara Y. Anticancer Agent-Induced Life-Threatening Skin Toxicities: A Database Study of Spontaneous Reporting Data. Oncologist, 24:266-272, 2019
47. Takahashi K, Yunokawa M, Sasada S, Takehara Y, Miyasaka N, Kato T, Tamura K. A novel prediction score for predicting the baseline risk of recurrence of stage I-II endometrial carcinoma. J Gynecol Oncol, 30:e8, 2019
48. Ito T, Kumagai Y, Itano K, Maruyama T, Tamura K, Kawasaki S, Suzuki T, Murakami Y. Mathematical analysis of gefitinib resistance of lung adenocarcinoma caused by MET amplification. Biochem Biophys Res Commun, 511:544-550, 2019
49. Turner NC, Alarcón E, Armstrong AC, Philco M, López Chuken YA, Sablin MP, Tamura K, Gómez Villanueva A, Pérez-Fidalgo JA, Cheung SYA, Corcoran C, Cullberg M, Davies BR, de Bruin EC, Foxley A, Lindemann JPO, Maudsley R, Moschetta M, Outhwaite E, Pass M, Rugman P, Schiavon G, Oliveira M. BEECH: a dose-finding run-in followed by a randomised phase II study assessing the efficacy of AKT inhibitor capivasertib (AZD5363) combined with paclitaxel in patients with estrogen receptor-positive advanced or metastatic breast cancer, and in a PIK3CA mutant sub-population. Ann Oncol, 30:774-780, 2019
50. Yap YS, Lu YS, Tamura K, Lee JE, Ko EY, Park YH, Cao AY, Lin CH, Toi M, Wu J, Lee SC. Insights Into Breast Cancer in the East vs the West: A Review. JAMA Oncol, doi: 10.1001/jamaoncol.2019.0620, 2019
51. Iwata H, Inoue K, Kaneko K, Ito Y, Tsugawa K, Hasegawa A, Nakagawa S, Kuratomi H, Tamura K. Subgroup analysis of Japanese patients in a Phase 3 study of atezolizumab in advanced triple-negative breast cancer (IMpassion130). Jpn J Clin Oncol, 49:1083-1091, 2019
52. Tamura K. Differences of cyclin-dependent kinase 4/6 inhibitor, palbociclib and abemaciclib, in breast cancer. Jpn J Clin Oncol, 49:993-998, 2019
53. Fujiwara H, Ushijima K, Nagao S, Takei Y, Shimada M, Takano M, Yoshino K, Kawano Y, Hirashima Y, Nagase S, Nishio S, Nishikawa T, Ito K, Shoji T, Kimura E, Takano T, Sugiyama T, Kigawa J, Fujiwara K, Suzuki M. A phase II randomized controlled study of pegylated liposomal doxorubicin and carboplatin vs. gemcitabine and carboplatin for platinum-sensitive recurrent ovarian cancer (GOTIC003/intergroup study). Int J Clin Oncol, 24:1284-1291, 2019
54. Okuma HS, Fujiwara Y. Have We Found the Key to Unravel Treatment Development Lags for Rare Cancers?: MASTER KEY Project. Clin Pharmacol Ther, 106:491-492, 2019
55. Wong KY, Fan C, Tanioka M, Parker JS, Nobel AB, Zeng D, Lin DY, Perou CM. I-Boost: an integrative boosting approach for predicting survival time with multiple genomics platforms. Genome Biol, 20:52, 2019
