Annual Report 2019
Department of Colorectal Surgery
Yukihide Kanemitsu, Shunsuke Tsukamoto, Konosuke Moritani, Yasuyuki Takamizawa
Introduction
The Department of Colorectal Surgery deals with colorectal cancer and allied malignancies in the colon and rectum. Liver metastasis from colorectal cancer is treated in cooperation with the Department of Hepatobiliary and Pancreatic Surgery. Lung metastasis from colorectal cancer is also treated in cooperation with the Department of Thoracic Surgery. Although surgery is still the main treatment modality for colorectal cancer, multidisciplinary treatments including radiotherapy and chemotherapy are important in advanced cancer. We have multidisciplinary meetings with the Department of Gastrointestinal Medical Oncology, the Department of Endoscopy, the Department of Diagnostic Radiology, and the Department of Pathology and Clinical Laboratories every week, and decide the treatment strategy with a multidisciplinary team (MDT) before treatment is performed.
The Team and What We Do
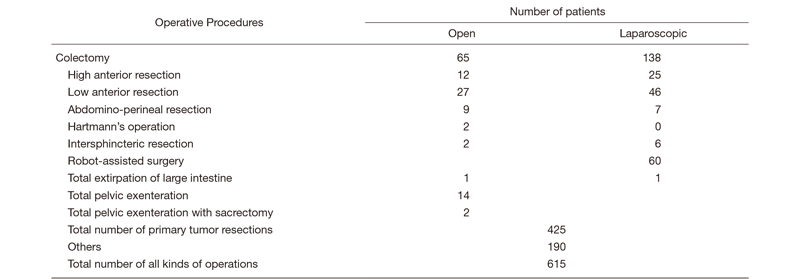
In the Department of Colorectal Surgery, five staff surgeons perform more than 600 colorectal operations per year, which is top of the class in Japan, and we always aim to improve the survival rate with safe surgery associated with less complications. The length of hospital stay is as short as seven days after surgery in laparotomy, laparoscopic surgery, and robot-assisted surgery, which allows more patients to receive surgical treatment with fewer beds.
There are five staff surgeons, one chief resident, and four to seven rotating residents. Every morning (7:30-8:30), we have a morning conference and rounds in wards 15A and B. An MDT meeting is held for cancer patients as a form of institutionalized communication every Tuesday morning (7:15-8:00), and a conference is held for the diagnosis of colorectal cancer: colorectal surgeons, endoscopists, and radiologists discuss the diagnosis for preoperative patients every Tuesday evening (18:30-19:30). Every Wednesday morning (7:00-7:30), a conference is held for the treatment of colorectal cancer: colorectal surgeons discuss treatments for preoperative and postoperative patients. Twelve to fifteen operations are performed a week in our department.
Robot-assisted surgery
In our department, we have been performing robot-assisted rectal surgeries, in which robotic surgery technology has been applied to rectal cancer treatment since March 2014. It is considered that rectal cancer surgery using the Da Vinci surgical system enables more intricate and precise operations because the doctor performs surgery with robotic support. It also enables safer and less invasive surgery since accurate three-dimensional image data can be obtained. The field of robot-assisted surgery is expected to play an important role in next-generation surgery. We have already confirmed the safety and feasibility of this new robot-assisted rectal surgery in the clinical study of 30 patients. Currently, the surgery is performed as a treatment option that is covered by insurance.
Research activities
Patients with advanced rectal cancers are treated with conventional surgery. Adjuvant chemotherapy is being used in stage III colorectal cancer patients in a clinical setting. Although preoperative radiotherapy is not performed routinely for advanced rectal cancer, patients with T4b rectal cancers or rectal cancers with multiple lymph node metastases are treated with preoperative chemoradiotherapy and surgery. Patients with symptoms caused by unresectable tumors are treated with palliative surgery including palliative resection, bypass, and stoma before chemotherapy. To evaluate the survival benefit and safety of primary resection plus chemotherapy compared to chemotherapy alone in asymptomatic stage IV colorectal cancer with synchronous unresectable metastatic disease, a randomized controlled trial comparing resection of primary tumor plus chemotherapy with chemotherapy alone in incurable stage IV colorectal cancer is ongoing (JCOG1007, iPACS). Another randomized controlled trial is ongoing to evaluate the non-inferiority of overall survival of laparoscopic surgery to open surgery for palliative resection of primary tumor in incurable stage IV colorectal cancer (JCOG1107, ENCORE). Symptomatic, stage IV colorectal cancer patients with non-curable metastasis are pre-operatively randomized to either open or laparoscopic colorectal resection. Patients with resectable liver metastasis are treated in cooperation with the Department of Hepatobiliary and Pancreatic Surgery and adjuvant chemotherapy regimens are being evaluated in a clinical trial (JCOG0603 study). To confirm the superiority of perioperative chemotherapy, a randomized phase II/III trial started in May 2015 comparing perioperative versus postoperative chemotherapy with modified infusional fluorouracil and folinic acid with oxaliplatin (mFOLFOX6) for lower rectal cancer patients with suspected lateral pelvic node metastasis (JCOG1310). The registration for JCOG1502C (a study to examine adjuvant chemotherapy for small bowel adenocarcinoma) and JCOG1503C (a study to examine the efficacy of aspirin for stage III colorectal cancer) began in 2017 and 2018, respectively. The registration for JCOG1801 (a study to examine neoadjuvant chemoradiotherapy for locally recurrent rectal cancer) began in 2019. In addition, a translational study (JCOG1506A1) to compare the clinical data from previous large-scale clinical trials including more than 4,000 patients and the genetic analysis data of clinical specimens such as surgical specimens and peripheral blood has been initiated in collaboration with the BioBank Japan (BBJ) toward improvement in treatment outcome and individualization of treatment. The registration for JCOG1805 (a study to examine adjuvant chemotherapy for stage II colorectal cancer patients at high risk of developing recurrence) began in 2020.
We also carry out basic research in cooperation with scientists at the National Cancer Center Research Institute (NCCRI) and the identification of a suitable treatment based on prediction is one of our important goals.
Clinical trials
Our department plays a central role in conducting multi-institutional clinical trials in Japan. Y. Kanemitsu is a representative of the Colorectal Cancer Group of the Japan Clinical Oncology Group (JCOG). Our department is participating in nine phase III JCOG studies.
1. JCOG0603: A randomized study that compares adjuvant modified FOLFOX (5-FU + l-LV + Oxaliplatin) to surgery alone after hepatic resection for liver metastasis from colorectal cancer. One hundred and seventy patients have been enrolled and recruitment continues.
2. JCOG1007: A randomized controlled trial comparing resection of primary tumor plus chemotherapy with chemotherapy alone in incurable stage IV colorectal cancer was terminated as of June 2019.
3. JCOG1018: Randomized phase III study of mFOLFOX7 or CAPOX plus bevacizumab versus 5-fluorouracil/leucovorin or capecitabine plus bevacizumab as first-line treatment in elderly patients with metastatic colorectal cancer is ongoing.
4. JCOG1107: A randomized controlled trial comparing laparoscopic surgery with open surgery in palliative resection of primary tumor in incurable stage IV colorectal cancer is ongoing.
5. JCOG1310: A phase II/III randomized controlled trial comparing perioperative versus postoperative chemotherapy with mFOLFOX6 for lower rectal cancer with suspected lateral pelvic node metastasis was terminated as of June 2019.
6. JCOG1410A: Japanese Observational Study to Evaluate the Accuracy of Preoperative Imaging Diagnosis for Lateral Pelvic Lymph Node Metastasis in Rectal Cancer is ongoing.
7. JCOG1506A: Prognostic or predictive biomarker study in patients who underwent surgery with/without postoperative chemotherapy for stage II/III colorectal cancer is ongoing.
8. JCOG1502C: A Global Study to Evaluate the Potential Benefit of Adjuvant Chemotherapy for Small Bowel Adenocarcinoma is ongoing.
9. JCOG1503C: Efficacy of aspirin for stage III colorectal cancer: a randomized double-blind placebo-controlled trial is ongoing.
10. JCOG1801: A randomized controlled trial comparing surgery plus adjuvant chemotherapy with preoperative chemoradiotherapy followed by surgery plus adjuvant chemotherapy for locally recurrent rectal cancer is ongoing.
11. JCOG1805: A randomized controlled trial to examine efficacy of adjuvant chemotherapy for stage II colorectal cancer patients at high risk of developing recurrence according to T-stage and three selected pathological factors.
Education
One senior resident and several residents were recruited to our department. Many foreign surgeons from different countries visited our department for training.
Future prospects
Although Japan has been leading the world in the development of new drugs such as irinotecan and oxaliplatin, it is far behind in the development of molecular targeted drugs. While the gap is being filled by international collaborative clinical trials, there is no guarantee that innovative new drugs will continue to dramatically improve treatment outcomes of cancer patients in the future. Therefore, it is a big but important challenge for clinical oncologists to think and act strategically. Outside of Japan, novel studies using the data from many clinical trials and the data from specimen examination have been carried out one after another. We believe that all the specialists involved in colorectal cancer treatment must work together with their wisdom and experience in order to improve treatment outcome. We will reconfirm the department’s research policy that a clinical question is reviewed by all participants and an answer is obtained from a randomized controlled trial, and continue to actively promote clinical trials in the future.
Development of a new laparoscopic surgical system using 8K Super Hi-Vision technology
At the National Cancer Center Hospital (NCCH), a national research project working on the development of a new laparoscopic surgical system and the utilization of high definition image data using 8K Super Hi-Vision technology has been initiated. With this project, further improvement of the treatment outcome of patients with colorectal cancer is expected in the future.
List of papers published in 2019
Journal
1. Moritani K, Kanemitsu Y, Shida D, Shitara K, Mizusawa J, Katayama H, Hamaguchi T, Shimada Y. A randomized controlled trial comparing primary tumour resection plus chemotherapy with chemotherapy alone in incurable stage IV colorectal cancer: JCOG1007 (iPACS study). Jpn J Clin Oncol, 50:89-93, 2020
2. Nakamura Y, Shida D, Tanabe T, Takamizawa Y, Imaizumi J, Ahiko Y, Sakamoto R, Moritani K, Tsukamoto S, Kanemitsu Y. Prognostic impact of preoperatively elevated and postoperatively normalized carcinoembryonic antigen levels following curative resection of stage I-III rectal cancer. Cancer Med, 9:653-662, 2020
3. Shida D, Kobayashi H, Kameyama M, Hase K, Maeda K, Suto T, Itabashi M, Funahashi K, Koyama F, Ozawa H, Noura S, Ishida H, Kanemitsu Y, Kotake K, Sugihara K. Factors affecting R0 resection of colorectal cancer with synchronous peritoneal metastases: a multicenter prospective observational study by the Japanese Society for Cancer of the Colon and Rectum. Int J Clin Oncol, 25:330-337, 2020
4. Tanabe T, Shida D, Tsukamoto S, Morizono G, Taniguchi H, Kanemitsu Y. Metachronous metastasis to inguinal lymph nodes from sigmoid colon adenocarcinoma with abdominal wall metastasis: a case report. BMC Cancer, 19:180, 2019
5. Nakamura Y, Shida D, Shibayama T, Yoshida A, Matsui Y, Shinoda Y, Iwata S, Kanemitsu Y. Giant multilocular prostatic cystadenoma. World J Surg Oncol, 17:42, 2019
6. Kitahara H, Honma Y, Ueno M, Kanemitsu Y, Ohkawa S, Mizusawa J, Furuse J, Shimada Y. Randomized phase III trial of post-operative chemotherapy for patients with stage I/II/III small bowel adenocarcinoma (JCOG1502C, J-BALLAD). Jpn J Clin Oncol, 49:287-290, 2019
7. Kanemitsu Y, Shida D, Tsukamoto S, Ueno H, Ishiguro M, Ishihara S, Komori K, Sugihara K. Nomograms predicting survival and recurrence in colonic cancer in the era of complete mesocolic excision. BJS Open, 3:539-548, 2019
8. Yoshida T, Shida D, Taniguchi H, Tsukamoto S, Kanemitsu Y. Long-Term Outcomes Following Partial Versus Complete Cystectomy in Advanced Colorectal Cancer with Regarding to the Extent of Bladder Invasion. Ann Surg Oncol, 26:1569-1576, 2019
9. Shida D, Tanabe T, Boku N, Takashima A, Yoshida T, Tsukamoto S, Kanemitsu Y. Prognostic Value of Primary Tumor Sidedness for Unresectable Stage IV Colorectal Cancer: A Retrospective Study. Ann Surg Oncol, 26:1358-1365, 2019
10. Kanemitsu Y. Robot-assisted laparoscopic surgery beyond total mesorectal excision for rectal cancer. Ann Laparosc Endosc Surg, 4:38, 2019
11. Komono A, Shida D, Iinuma G, Tsukamoto S, Sakamoto R, Moritani K, Miyake M, Kanemitsu Y. Preoperative T staging of colon cancer using CT colonography with multiplanar reconstruction: new diagnostic criteria based on “bordering vessels”. Int J Colorectal Dis, 34:641-648, 2019
12. Shida D, Kanemitsu Y, Hamaguchi T, Shimada Y. Introducing the eighth edition of the tumor-node-metastasis classification as relevant to colorectal cancer, anal cancer and appendiceal cancer: a comparison study with the seventh edition of the tumor-node-metastasis and the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma. Jpn J Clin Oncol, 49:321-328, 2019
13. Imaizumi J, Shida D, Narita Y, Miyakita Y, Tanabe T, Takashima A, Boku N, Igaki H, Itami J, Kanemitsu Y. Prognostic factors of brain metastases from colorectal cancer. BMC Cancer, 19:755, 2019
14. Tanabe T, Shida D, Komukai S, Nakamura Y, Tsukamoto S, Kanemitsu Y. Long-term outcomes after surgical dissection of inguinal lymph node metastasis from rectal or anal canal adenocarcinoma. BMC Cancer, 19:733, 2019
15. Watanabe J, Shoji H, Hamaguchi T, Miyamoto T, Hirano H, Iwasa S, Honma Y, Takashima A, Kato K, Ito Y, Itami J, Kanemitsu Y, Boku N. Chemoradiotherapy for Local Recurrence of Rectal Cancer: A Single Center Study of 18 Patients. In Vivo, 33:1363-1368, 2019
16. Miyamoto K, Takashima A, Mizusawa J, Sato Y, Shimada Y, Katayama H, Nakamura K, Shibata T, Fukuda H, Shida D, Kanemitsu Y, Hamaguchi T. Efficacy of aspirin for stage III colorectal cancer: a randomized double-blind placebo-controlled trial (JCOG1503C, EPISODE-III trial). Jpn J Clin Oncol, 49:985-990, 2019
17. Shida D, Boku N, Tanabe T, Yoshida T, Tsukamoto S, Takashima A, Kanemitsu Y. Primary Tumor Resection for Stage IV Colorectal Cancer in the Era of Targeted Chemotherapy. J Gastrointest Surg, 23:2144-2150, 2019
18. Ahiko Y, Shida D, Horie T, Tanabe T, Takamizawa Y, Sakamoto R, Moritani K, Tsukamoto S, Kanemitsu Y. Controlling nutritional status (CONUT) score as a preoperative risk assessment index for older patients with colorectal cancer. BMC Cancer, 19:946, 2019
19. Shida D. ASO Author Reflections: Prognostic Impact of Primary Tumor Sidedness for Unresectable Stage IV Colorectal Cancer. Ann Surg Oncol, 26:666-667, 2019
