Annual Report 2019
Department of Gastrointestinal Medical Oncology
Narikazu Boku, Ken Kato, Atsuo Takashima, Satoru Iwasa, Yoshitaka Honma, Hirokazu Shoji, Natsuko Okita, Hidekazu Hirano, Shun Yamamoto, Kotoe Oshima
Introduction
The Department of Gastrointestinal Medical Oncology focuses on treatment, development of new drugs and establishment of new standard chemotherapy regimens including multi-modality treatment with surgery and/or radiotherapy for esophageal/gastric/colorectal/gastrointestinal stromal tumor (GIST), and other gastrointestinal (GI) malignancies.
The Team and What We Do
The staff of our division consists of eight medical oncologists, two chief residents, and three or four rotating residents. We have a daily case conference every evening for discussing each patient’s treatment and a weekly research conference for sharing and discussing the progress of clinical studies and/or translational research. Multi-disciplinary team meetings with the surgical divisions (Colorectal, Gastric and Esophageal Surgery Divisions) and the Radiation Oncology Division are held weekly to decide optimal treatment strategies for each patient and to discuss treatment consensus for each disease. We treated 62.8 hospitalized patients/day (22,979/year) and 97.0 outpatients/day (23,572/year) including 875 newly diagnosed patients in 2019.
Research activities
We are putting a lot of work into developing clinical research to establish new treatments and answer clinical questions. As for the late phase clinical studies, we are playing a leading role in JCOG (Japan Clinical Oncology Group) and WJOG (West Japan Oncology Group), which are the largest cooperative study groups for cancer treatment in Japan. As an early phase clinical study, in collaboration with other active hospitals, we are conducting investigator-initiated trials using unapproved drugs under the regulation of the GCP (Good Clinical Practice). Moreover, we collaborate with the National Cancer Center Research Institute and other distinguished basic research institutions to make a clinical research based on our own original new ideas and findings. We published 49 English articles in 2019.
Clinical trials
We are participating many clinical trials in collaboration with the Surgery and Radiation Oncology Divisions in our hospital or other institutes including JCOG, WJOG, company-initiated trials, and other collaborative investigator-initiated trials and in-house clinical trials. A total of 217 patients were enrolled in 2019 (Table).
Table. Major Clinical Trials (Esophageal, Stomach, and Colorectal Cancer)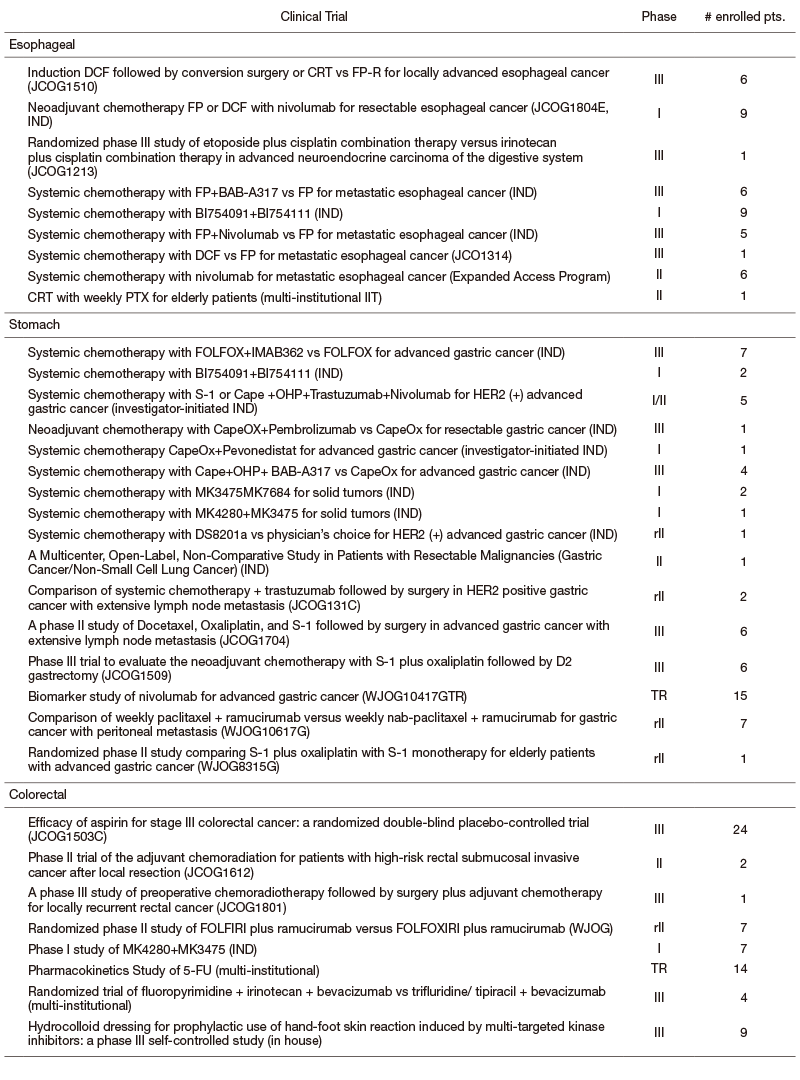

Education
As on-the-job training, the staff trains the residents about management of gastrointestinal malignancies, and instructed about the clinical research by giving them research themes. There are 15 English papers written by residents as the first author in 2019. The chief residents can learn basic research in collaboration with the National Cancer Center Research Institute.
Future prospects
Our division focus on “clinical practice”, “education” and “clinical research” to improve gastrointestinal cancer treatment. For daily practice, we give the patients the optimal treatment; moreover, we will always pay attention to improving patient care to satisfy unmet clinical needs. For education, we will keep educating the residents and chief residents; the staff doctors will also strive to keep improving themselves. For the clinical research, we will strive toward the next step of clinical development to establish new global standard treatments.
List of papers published in 2019
Journal
1. Yamamoto Y, Kondo S, Matsuzaki J, Esaki M, Okusaka T, Shimada K, Murakami Y, Enomoto M, Tamori A, Kato K, Aoki Y, Takizawa S, Sakamoto H, Niida S, Takeshita F, Ochiya T. Highly Sensitive Circulating MicroRNA Panel for Accurate Detection of Hepatocellular Carcinoma in Patients With Liver Disease. Hepatol Commun, 4:284-297, 2020
2. Honma Y, Nagashima K, Hirano H, Shoji H, Iwasa S, Takashima A, Okita N, Kato K, Boku N, Murakami N, Inaba K, Ito Y, Itami J, Kanamori J, Oguma J, Daiko H. Clinical outcomes of locally advanced esophageal neuroendocrine carcinoma treated with chemoradiotherapy. Cancer Med, 9:595-604, 2020
3. Kadota T, Abe S, Yoda Y, Yoshinaga S, Oda I, Kojima T, Kato K, Daiko H, Yano T. Clinical outcomes according to the modified endoscopic criteria for neoadjuvant chemotherapy in resectable esophageal squamous cell carcinoma. Dig Endosc, 32:337-345, 2020
4. Satomi-Tsushita N, Honma Y, Nagashima K, Ito Y, Hirano H, Shoji H, Takashima A, Iwasa S, Kato K, Hamaguchi T, Itami J, Boku N. Risk Factors of Severe Benign Cicatricial Stricture After Definitive Chemoradiation for Localized T3 Esophageal Carcinoma. Anticancer Res, 40:1071-1077, 2020
5. Sudo K, Kato K, Matsuzaki J, Takizawa S, Aoki Y, Shoji H, Iwasa S, Honma Y, Takashima A, Sakamoto H, Naka T, Sekine S, Boku N, Ochiya T. Identification of serum microRNAs predicting the response of esophageal squamous-cell carcinoma to nivolumab. Jpn J Clin Oncol, 50:114-121, 2020
6. Murakami N, Mori T, Kubo Y, Yoshimoto S, Ito K, Honma Y, Ueno T, Kobayashi K, Okamoto H, Boku N, Takahashi K, Inaba K, Okuma K, Igaki H, Nakayama Y, Itami J. Prognostic impact of immunohistopathologic features in definitive radiation therapy for nasopharyngeal cancer patients. J Radiat Res, 61:161-168, 2020
7. Asakura K, Kadota T, Matsuzaki J, Yoshida Y, Yamamoto Y, Nakagawa K, Takizawa S, Aoki Y, Nakamura E, Miura J, Sakamoto H, Kato K, Watanabe SI, Ochiya T. A miRNA-based diagnostic model predicts resectable lung cancer in humans with high accuracy. Commun Biol, 3:134, 2020
8. Shitara K, Honma Y, Omuro Y, Yamaguchi K, Chin K, Muro K, Nakagawa S, Kawakami S, Hironaka S, Nishina T. Efficacy of trastuzumab emtansine in Japanese patients with previously treated HER2-positive locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma: A subgroup analysis of the GATSBY study. Asia Pac J Clin Oncol, 16:5-13, 2020
9. Suzuki T, Sukawa Y, Imamura CK, Masuishi T, Satake H, Kumekawa Y, Funakoshi S, Kotaka M, Horie Y, Kawai S, Okuda H, Terazawa T, Kondoh C, Kato K, Yoshimura K, Ishikawa H, Hamamoto Y, Boku N, Takaishi H, Kanai T. A Phase II Study of Regorafenib With a Lower Starting Dose in Patients With Metastatic Colorectal Cancer: Exposure-Toxicity Analysis of Unbound Regorafenib and Its Active Metabolites (RESET Trial). Clin Colorectal Cancer, 19:13-21.e3, 2020
10. Nakajima TE, Boku N, Doi A, Arai H, Mizukami T, Horie Y, Izawa N, Hirakawa M, Ogura T, Tsuda T, Sunakawa Y. Phase I study of the anti-heparin-binding epidermal growth factor-like growth factor antibody U3-1565 with cetuximab in patients with cetuximab- or panitumumab-resistant metastatic colorectal cancer. Invest New Drugs, 38:410-418, 2020
11. Ishikawa M, Iwasa S, Nagashima K, Aoki M, Imazeki H, Hirano H, Shoji H, Honma Y, Okita N, Takashima A, Kato K, Saruta M, Boku N. Retrospective comparison of nab-paclitaxel plus ramucirumab and paclitaxel plus ramucirumab as second-line treatment for advanced gastric cancer focusing on peritoneal metastasis. Invest New Drugs, 38:533-540, 2020
12. Satoh T, Kang YK, Chao Y, Ryu MH, Kato K, Cheol Chung H, Chen JS, Muro K, Ki Kang W, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Tanimoto M, Chen LT, Boku N. Exploratory subgroup analysis of patients with prior trastuzumab use in the ATTRACTION-2 trial: a randomized phase III clinical trial investigating the efficacy and safety of nivolumab in patients with advanced gastric/gastroesophageal junction cancer. Gastric Cancer, 23:143-153, 2020
13. Yano T, Hasuike N, Ono H, Boku N, Ogawa G, Kadota T, Oda I, Doyama H, Hori S, Iishi H, Takahashi A, Takizawa K, Muto M. Factors associated with technical difficulty of endoscopic submucosal dissection for early gastric cancer that met the expanded indication criteria: post hoc analysis of a multi-institutional prospective confirmatory trial (JCOG0607). Gastric Cancer, 23:168-174, 2020
14. Wagner AD, Lordick F, Grabsch HI, Terashima M, Terada M, Yoshikawa T, Boku N, Kataoka K, Smyth EC, Mauer M, Haustermans K, Moehler MH. Multidisciplinary management of stage II-III gastric and gastro-oesophageal junction cancer. Eur J Cancer, 124:67-76, 2020
15. Abe Y, Hirano H, Shoji H, Tada A, Isoyama J, Kakudo A, Gunji D, Honda K, Boku N, Adachi J, Tomonaga T. Comprehensive characterization of the phosphoproteome of gastric cancer from endoscopic biopsy specimens. Theranostics, 10:2115-2129, 2020
16. Doi T, Boku N, Onozawa Y, Takahashi K, Kawaguchi O, Ohtsu A. Phase I dose-escalation study of the safety, tolerability, and pharmacokinetics of aflibercept in combination with S-1 in Japanese patients with advanced solid malignancies. Invest New Drugs, 2020
17. Yamaguchi T, Takashima A, Nagashima K, Makuuchi R, Aizawa M, Ohashi M, Tashiro K, Yamada T, Kinoshita T, Hata H, Kawachi Y, Kawabata R, Tsuji T, Hihara J, Sakamoto T, Fukagawa T, Katai H, Higuchi K, Boku N. Efficacy of Postoperative Chemotherapy After Resection that Leaves No Macroscopically Visible Disease of Gastric Cancer with Positive Peritoneal Lavage Cytology (CY1) or Localized Peritoneum Metastasis (P1a): A Multicenter Retrospective Study. Ann Surg Oncol, 27:284-292, 2020
18. Okamura Y, Yasukawa S, Narimatsu H, Boku N, Fukutomi A, Konishi M, Morinaga S, Toyama H, Kaneoka Y, Shimizu Y, Nakamori S, Sata N, Yamakita K, Takahashi A, Kainuma O, Hishinuma S, Yamaguchi R, Nagino M, Hirano S, Yanagisawa A, Mori K, Uesaka K. Human equilibrative nucleoside transporter-1 expression is a predictor in patients with resected pancreatic cancer treated with adjuvant S-1 chemotherapy. Cancer Sci, 111:548-560, 2020
19. Sato Y, Kurokawa Y, Doki Y, Mizusawa J, Tanaka K, Katayama H, Boku N, Yoshikawa T, Terashima M. A Phase II study of preoperative chemotherapy with docetaxel, oxaliplatin and S-1 in gastric cancer with extensive lymph node metastasis (JCOG1704). Future Oncol, 16:31-38, 2020
20. Yokota T, Kato K, Hamamoto Y, Tsubosa Y, Ogawa H, Ito Y, Hara H, Ura T, Kojima T, Chin K, Hironaka S, Kii T, Kojima Y, Akutsu Y, Matsushita H, Kawakami K, Mori K, Makiuchi T, Nagumo R, Kitagawa Y. A 3-Year Overall Survival Update From a Phase 2 Study of Chemoselection With DCF and Subsequent Conversion Surgery for Locally Advanced Unresectable Esophageal Cancer. Ann Surg Oncol, 27:460-467, 2020
21. Nakajima TE, Yamaguchi K, Boku N, Hyodo I, Mizusawa J, Hara H, Nishina T, Sakamoto T, Shitara K, Shinozaki K, Katayama H, Nakamura S, Muro K, Terashima M. Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer, 23:677-688, 2020
22. Sunami E, Kusumoto T, Ota M, Sakamoto Y, Yoshida K, Tomita N, Maeda A, Teshima J, Okabe M, Tanaka C, Yamauchi J, Itabashi M, Kotake K, Takahashi K, Baba H, Boku N, Aiba K, Ishiguro M, Morita S, Takenaka N, Okude R, Sugihara K. S-1 and Oxaliplatin Versus Tegafur-uracil and Leucovorin as Postoperative Adjuvant Chemotherapy in Patients With High-risk Stage III Colon Cancer (ACTS-CC 02): A Randomized, Open-label, Multicenter, Phase III Superiority Trial. Clin Colorectal Cancer, 19:22-31.e6, 2020
23. Sunami K, Ichikawa H, Kubo T, Kato M, Fujiwara Y, Shimomura A, Koyama T, Kakishima H, Kitami M, Matsushita H, Furukawa E, Narushima D, Nagai M, Taniguchi H, Motoi N, Sekine S, Maeshima A, Mori T, Watanabe R, Yoshida M, Yoshida A, Yoshida H, Satomi K, Sukeda A, Hashimoto T, Shimizu T, Iwasa S, Yonemori K, Kato K, Morizane C, Ogawa C, Tanabe N, Sugano K, Hiraoka N, Tamura K, Yoshida T, Fujiwara Y, Ochiai A, Yamamoto N, Kohno T. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci, 110:1480-1490, 2019
24. Imaizumi J, Shida D, Narita Y, Miyakita Y, Tanabe T, Takashima A, Boku N, Igaki H, Itami J, Kanemitsu Y. Prognostic factors of brain metastases from colorectal cancer. BMC Cancer, 19:755, 2019
25. Watanabe J, Shoji H, Hamaguchi T, Miyamoto T, Hirano H, Iwasa S, Honma Y, Takashima A, Kato K, Ito Y, Itami J, Kanemitsu Y, Boku N. Chemoradiotherapy for Local Recurrence of Rectal Cancer: A Single Center Study of 18 Patients. In Vivo, 33:1363-1368, 2019
26. Miyamoto K, Takashima A, Mizusawa J, Sato Y, Shimada Y, Katayama H, Nakamura K, Shibata T, Fukuda H, Shida D, Kanemitsu Y, Hamaguchi T. Efficacy of aspirin for stage III colorectal cancer: a randomized double-blind placebo-controlled trial (JCOG1503C, EPISODE-III trial). Jpn J Clin Oncol, 49:985-990, 2019
27. Sudo K, Kato K, Matsuzaki J, Boku N, Abe S, Saito Y, Daiko H, Takizawa S, Aoki Y, Sakamoto H, Niida S, Takeshita F, Fukuda T, Ochiya T. Development and Validation of an Esophageal Squamous Cell Carcinoma Detection Model by Large-Scale MicroRNA Profiling. JAMA Netw Open, 2:e194573, 2019
28. Terada M, Hara H, Daiko H, Mizusawa J, Kadota T, Hori K, Ogawa H, Ogata T, Sakanaka K, Sakamoto T, Kato K, Kitagawa Y. Phase III study of tri-modality combination therapy with induction docetaxel plus cisplatin and 5-fluorouracil versus definitive chemoradiotherapy for locally advanced unresectable squamous-cell carcinoma of the thoracic esophagus (JCOG1510: TRIANgLE). Jpn J Clin Oncol, 49:1055-1060, 2019
29. Nambu M, Masuda T, Ito S, Kato K, Kojima T, Daiko H, Ito Y, Honda K, Ohtsuki S. Leucine-Rich Alpha-2-Glycoprotein 1 in Serum Is a Possible Biomarker to Predict Response to Preoperative Chemoradiotherapy for Esophageal Cancer. Biol Pharm Bull, 42:1766-1771, 2019
30. Aoki M, Shoji H, Nagashima K, Imazeki H, Miyamoto T, Hirano H, Honma Y, Iwasa S, Okita N, Takashima A, Kato K, Higuchi K, Boku N. Hyperprogressive disease during nivolumab or irinotecan treatment in patients with advanced gastric cancer. ESMO Open, 4:e000488, 2019
31. Arai H, Iwasa S, Boku N, Kawahira M, Yasui H, Masuishi T, Muro K, Minashi K, Hironaka S, Fukuda N, Takahari D, Nakajima TE. Fluoropyrimidine with or without platinum as first-line chemotherapy in patients with advanced gastric cancer and severe peritoneal metastasis: a multicenter retrospective study. BMC Cancer, 19:652, 2019
32. Ito T, Honma Y, Hirano H, Shoji H, Okita N, Iwasa S, Takashima A, Kato K, Boku N. S-1 Monotherapy After Failure of Platinum Plus 5-Fluorouracil Chemotherapy in Recurrent or Metastatic Esophageal Carcinoma. Anticancer Res, 39:3931-3936, 2019
33. Masuda K, Shoji H, Nagashima K, Yamamoto S, Ishikawa M, Imazeki H, Aoki M, Miyamoto T, Hirano H, Honma Y, Iwasa S, Okita N, Takashima A, Kato K, Boku N. Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer, 19:974, 2019
34. Monma S, Kato K, Shouji H, Okita N, Takashima A, Honma Y, Iwasa S, Hamaguchi T, Yamada Y, Shimada Y, Boku N, Nagashima K, Ito Y, Itami J. Gastric mucosal injury and hemorrhage after definitive chemoradiotherapy for locally advanced esophageal cancer. Esophagus, 16:402-407, 2019
35. Nagata Y, Sawada R, Takashima A, Shoji H, Honma Y, Iwasa S, Amano K, Kato K, Hamaguchi T, Shimada Y, Saruta M, Boku N. Efficacy and safety of pemetrexed plus cisplatin as first-line chemotherapy in advanced malignant peritoneal mesothelioma. Jpn J Clin Oncol, 49:1004-1008, 2019
36. Yamada Y, Boku N, Mizusawa J, Iwasa S, Kadowaki S, Nakayama N, Azuma M, Sakamoto T, Shitara K, Tamura T, Chin K, Hata H, Nakamori M, Hara H, Yasui H, Katayama H, Fukuda H, Yoshikawa T, Sasako M, Terashima M. Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): an open-label, phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol, 4:501-510, 2019
37. Murakami N, Mori T, Nakamura S, Yoshimoto S, Honma Y, Ueno T, Kobayashi K, Kashihara T, Takahashi K, Inaba K, Okuma K, Igaki H, Nakayama Y, Itami J. Prognostic value of the expression of epithelial cell adhesion molecules in head and neck squamous cell carcinoma treated by definitive radiotherapy. J Radiat Res, 60:803-811, 2019
38. Hirano H, Shimizu C, Kawachi A, Ozawa M, Higuchi A, Yoshida S, Shimizu K, Tatara R, Horibe K. Preferences Regarding End-of-Life Care Among Adolescents and Young Adults With Cancer: Results From a Comprehensive Multicenter Survey in Japan. J Pain Symptom Manage, 58:235-243.e1, 2019
39. Kono T, Imanishi N, Nozawa K, Takashima A, Maheswari RU, Gonome H, Yamada J. Optical characteristics of human skin with hyperpigmentation caused by fluorinated pyrimidine anticancer agent. Biomed Opt Express, 10:3747-3759, 2019
40. Nakano MH, Udagawa C, Shimo A, Kojima Y, Yoshie R, Zaha H, Abe N, Motonari T, Unesoko M, Tamura K, Shimoi T, Yoshida M, Yoshida T, Sakamoto H, Kato K, Mushiroda T, Tsugawa K, Zembutsu H. A Genome-Wide Association Study Identifies Five Novel Genetic Markers for Trastuzumab-Induced Cardiotoxicity in Japanese Population. Biol Pharm Bull, 42:2045-2053, 2019
41. Hirano H, Kato K. Systemic treatment of advanced esophageal squamous cell carcinoma: chemotherapy, molecular-targeting therapy and immunotherapy. Jpn J Clin Oncol, 49:412-420, 2019
42. Tomita N, Kunieda K, Maeda A, Hamada C, Yamanaka T, Sato T, Yoshida K, Boku N, Nezu R, Yamaguchi S, Mishima H, Sadahiro S, Muro K, Ishiguro M, Sakamoto J, Saji S, Maehara Y. Phase III randomised trial comparing 6 vs. 12-month of capecitabine as adjuvant chemotherapy for patients with stage III colon cancer: final results of the JFMC37-0801 study. Br J Cancer, 120:689-696, 2019
43. Onidani K, Shoji H, Kakizaki T, Yoshimoto S, Okaya S, Miura N, Sekikawa S, Furuta K, Lim CT, Shibahara T, Boku N, Kato K, Honda K. Monitoring of cancer patients via next-generation sequencing of patient-derived circulating tumor cells and tumor DNA. Cancer Sci, 110:2590-2599, 2019
44. Satomi-Tsushita N, Shimomura A, Matsuzaki J, Yamamoto Y, Kawauchi J, Takizawa S, Aoki Y, Sakamoto H, Kato K, Shimizu C, Ochiya T, Tamura K. Serum microRNA-based prediction of responsiveness to eribulin in metastatic breast cancer. PLoS One, 14:e0222024, 2019
45. Takahari D, Chin K, Ishizuka N, Takashima A, Minashi K, Kadowaki S, Nishina T, Nakajima TE, Amagai K, Machida N, Goto M, Taku K, Wakatsuki T, Shoji H, Hironaka S, Boku N, Yamaguchi K. Multicenter phase II study of trastuzumab with S-1 plus oxaliplatin for chemotherapy-na?ve, HER2-positive advanced gastric cancer. Gastric Cancer, 22:1238-1246, 2019
46. Zhao H, Koyanagi K, Kato K, Ito Y, Itami J, Igaki H, Tachimori Y. Comparison of long-term outcomes between radical esophagectomy and definitive chemoradiotherapy in patients with clinical T1bN0M0 esophageal squamous cell carcinoma. J Thorac Dis, 11:4654-4662, 2019
47. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol, 20:1506-1517, 2019
48. Ohno M, Matsuzaki J, Kawauchi J, Aoki Y, Miura J, Takizawa S, Kato K, Sakamoto H, Matsushita Y, Takahashi M, Miyakita Y, Ichimura K, Narita Y, Ochiya T. Assessment of the Diagnostic Utility of Serum MicroRNA Classification in Patients With Diffuse Glioma. JAMA Netw Open, 2:e1916953, 2019
49. Yamazawa E, Honma Y, Satomi K, Taniguchi H, Takahashi M, Yoshida A, Tominaga K, Miyakita Y, Ohno M, Asanome T, Satomi N, Narita Y. A rare case of brain metastasis from poorly differentiated small bowel adenocarcinoma. Surg Neurol Int, 10:256, 2019
