Annual Report 2019
Department of Hepatobiliary and Pancreatic Oncology
Takuji Okusaka, Chigusa Morizane, Susumu Hijioka, Shunsuke Kondo, Yasunari Sakamoto, Akihiro Ohba, Yoshikuni Nagashio, Yuta Maruki, Yuya Hisada, Motohiro Yoshinari
Introduction
The Department of Hepatobiliary and Pancreatic Oncology treats tumors originating from the liver, biliary system or pancreas, which include hepatocellular carcinoma (HCC), biliary tract cancer and pancreatic cancer. As part of the multi-disciplinary care given at the National Cancer Center Hospital (NCCH), we work closely with surgeons and radiologists who have special expertise in these areas. We also conduct clinical and translational researches for hepatobiliary and pancreatic tumors and seek to develop new and more effective diagnostic methods and treatments.
The Team and What We Do
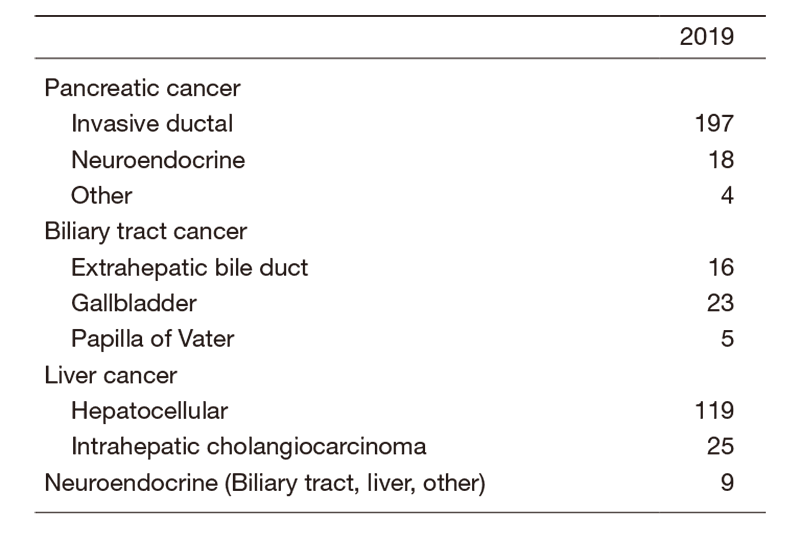
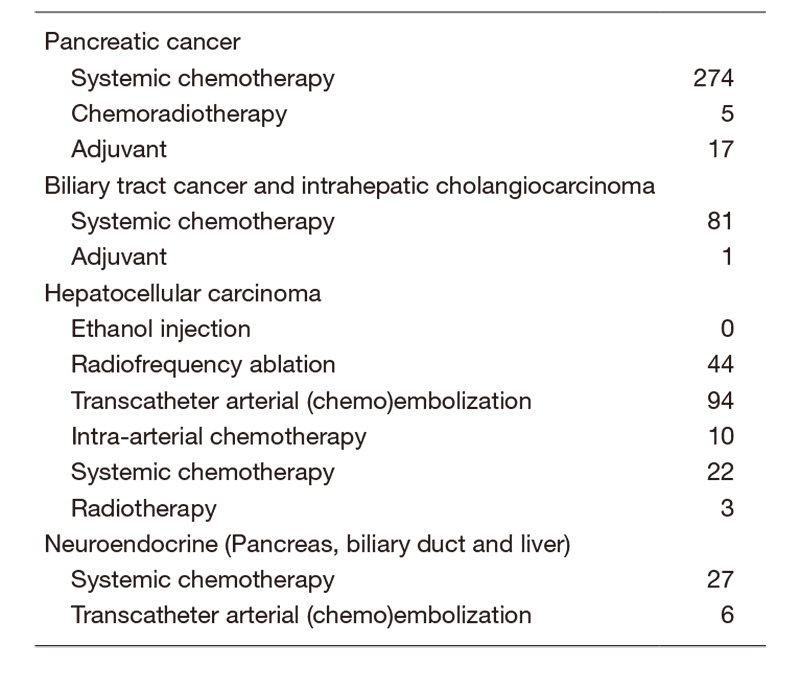
The Department consists of seven staff oncologists and several residents. We have used percutaneous ablation therapy for most patients with three or fewer HCC nodules, all of which are smaller than 3 cm in diameter. We also perform transcatheter arterial chemoembolization (TACE), mainly in patients with multiple HCC nodules. Systemic or intra-arterial chemotherapeutic regimens are indicated in advanced HCC patients for whom locoregional intervention and surgery are unsuitable or had been unsuccessful. In patients with unresectable pancreatic cancer or biliary tract cancer, chemotherapy is performed in clinical practice or as a clinical trial to develop new treatment. We have actively introduced endoscopic procedures for imaging diagnosis (endoscopic ultrasonography: EUS, endoscopic retrograde cholangiopancreatography: ERCP), tumor biopsy (EUS-guided fine needle aspiration: EUS-FNA), and biliary drainage including EUS-guided hepaticogastrostomy (EUS-HGS) and EUS-guided choledochoduodenostomy (EUS-CDS) (Tables 1 & 2).
Research activities
We published 10 papers as a first author in peer-reviewed journals in 2019.
The prevalence of germline mutations in patients with biliary tract carcinoma (BTC) remains unclear. Terashima et al. investigated the prevalence and types of germline mutations in patients with BTC. They reviewed 269 patients with pathologically proven BTC and collected clinical characteristics, including medical and family histories. Additionally, they evaluated germline variants in 21 genes associated with hereditary predisposition for cancer by targeted sequencing in patients meeting ≥1 of the following criteria: 1) hereditary breast and/or ovarian cancer (HBOC) testing criteria modified for BTC, 2) Revised Bethesda Guidelines (RBGs) modified for BTC (modified RBG), 3) familial BTC criteria, or 4) young BTC criteria. Among the 269 patients, 80 met at least one criterion. Three pathogenic mutations in three patients were identified: two in BRCA2 and one in BRCA1. Among the 16 patients meeting modified HBOC testing criteria, 2 harbored germline BRCA2 mutations, and 1 harbored a germline BRCA1 mutation. However, no mutation in mismatch-repair genes was detected, despite 63 patients meeting modified RBG screening criteria and 18 qualifying as young BTC patients. We detected high prevalence of pathogenic germline mutations in BRCA1/2 and none in mismatch-repair genes in BTC patients following enrichment according to family or medical history in this study.
Three or more stents may be needed in patients with extensive stricturing in Bismuth type IIIa/IV hilar malignant strictures. Partial stent-in-stent (PSIS) deployment has been the primary intervention for hilar malignant biliary stricture (MBS). However, simultaneous side-by-side (SBS) stent placement has become feasible with the development of the <6F diameter stent delivery system. Maruki et al. assessed the efficacy and safety of a new hybrid method combining PSIS and SBS stent placement for trisegment biliary drainage. This study included 17 consecutive patients with Bismuth IIIa or IV malignant strictures who underwent endoscopic drainage using the hybrid method. Diameters of the delivery stents were 5.4F (n = 10) and 5.7F (n = 7). The technical success rate was 82% (14/17), and the median length of procedures was 54 minutes. Two patients required predilatation for deployment of the third self-expandable metallic stent through the mesh of the first deployed stent. Two patients (12%) developed cholecystitis as early adverse events, and 1 patient (6%) developed liver abscess as a late adverse event. The time to recurrent biliary obstruction among those with successful initial trisegmental drainage was 189 days (95% confidence interval, 124-254). The hybrid method for unresectable hilar MBS is an effective endoscopic drainage method, and the ease of these procedures is partly attributed to the thinner stent delivery system.
Clinical trials
27 clinical trials are ongoing, including two phase I trials, eight phase II trials, four phase III trials such as adjuvant chemotherapy after resection versus resection alone for patients with resectable tumors, and chemotherapy with a new regimen versus standard therapy for patients with advanced tumors, and several observation or registration trials. Our studies are supported by the National Cancer Center Research and Development Fund (Grant No. 29-A-3), Project for Development of Innovative Research on Cancer Therapeutics (19ck0106350h0003, 19ck0106355h0003, 19ck0106354h0003) from the Japan Agency for Medical Research and Development.
Education
Our staff members are working closely with residents to support their skill development and knowledge expansion in both clinical and research fields. We are conducting conferences daily for clinical practice and weekly for research development. The residents in our department published six papers as a first author in peer-reviewed journals in 2019, and are performing 6 planning or ongoing studies as a leading researcher, with assistance from staff members.
Future prospects
Our department keeps providing best and latest diagnosis, treatment and supportive care, and developing their more effective methods and techniques for all patients with hepatobiliary and pancreatic cancer in this country and all over the world. Among them, conducting clinical trials with novel promising agents for this disease is considered one of the most important tasks, and establishment of cutting-edge endoscopic procedures in this field is the most significant mission for us.
List of papers published in 2019
Journal
1. Ohmoto A, Morizane C. Genomic Profiles and Current Therapeutic Agents in Neuroendocrine Neoplasms. Curr Drug Targets, 21:389-405, 2020
2. Yamamoto Y, Kondo S, Matsuzaki J, Esaki M, Okusaka T, Shimada K, Murakami Y, Enomoto M, Tamori A, Kato K, Aoki Y, Takizawa S, Sakamoto H, Niida S, Takeshita F, Ochiya T. Highly Sensitive Circulating MicroRNA Panel for Accurate Detection of Hepatocellular Carcinoma in Patients With Liver Disease. Hepatol Commun, 4:284-297, 2020
3. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut, 69:1492-1501, 2020
4. Okusaka T, Nakamura M, Yoshida M, Kitano M, Uesaka K, Ito Y, Furuse J, Hanada K, Okazaki K. Clinical Practice Guidelines for Pancreatic Cancer 2019 From the Japan Pancreas Society: A Synopsis. Pancreas, 49:326-335, 2020
5. Nagashio Y, Hijioka S, Kanai Y, Ohba A, Maruki Y, Okusaka T, Saito Y. Novel side-by-side metal stent placement for recurrent hepatic hilar obstruction after placement of multiple metal stents. Endoscopy, 2020
6. Maruki Y, Hijioka S, Wu SYS, Ohba A, Nagashio Y, Kondo S, Morizane C, Ueno H, Okusaka T, Saito Y. Novel endoscopic technique for trisegment drainage in patients with unresectable hilar malignant biliary strictures (with video). Gastrointest Endosc, 2020
7. Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, Aikata H, Kawaguchi Y, Wada Y, Numata K, Inaba Y, Kuromatsu R, Kobayashi M, Okusaka T, Tamai T, Kitamura C, Saito K, Haruna K, Okita K, Kumada H. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol, 55:113-122, 2020
8. Hirono S, Shimizu Y, Ohtsuka T, Kin T, Hara K, Kanno A, Koshita S, Hanada K, Kitano M, Inoue H, Itoi T, Ueki T, Shimokawa T, Hijioka S, Yanagisawa A, Nakamura M, Okazaki K, Yamaue H. Recurrence patterns after surgical resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas; a multicenter, retrospective study of 1074 IPMN patients by the Japan Pancreas Society. J Gastroenterol, 55:86-99, 2020
9. Ohtsuka T, Nakamura M, Hijioka S, Shimizu Y, Unno M, Tanabe M, Nagakawa Y, Takaori K, Hirono S, Gotohda N, Kimura W, Ito K, Katanuma A, Sano T, Urata T, Kita E, Hanada K, Tada M, Aoki T, Serikawa M, Okamoto K, Isayama H, Gotoh Y, Ishigami K, Yamaguchi H, Yamao K, Sugiyama M, Okazaki K. Prediction of the Probability of Malignancy in Mucinous Cystic Neoplasm of the Pancreas With Ovarian-Type Stroma: A Nationwide Multicenter Study in Japan. Pancreas, 49:181-186, 2020
10. Okuyama H, Ikeda M, Okusaka T, Furukawa M, Ohkawa S, Hosokawa A, Kojima Y, Hara H, Murohisa G, Shioji K, Asagi A, Mizuno N, Kojima M, Yamanaka T, Furuse J. A phase II trial of everolimus in patients with advanced pancreatic neuroendocrine carcinoma refractory or intolerant to platinum-containing chemotherapy (NECTOR trial). Neuroendocrinology, 2020
11. Kudo M, Okusaka T, Motomura K, Ohno I, Morimoto M, Seo S, Wada Y, Sato S, Yamashita T, Furukawa M, Aramaki T, Nadano S, Ohkawa K, Fujii H, Kudo T, Furuse J, Takai H, Homma G, Yoshikawa R, Zhu AX. Ramucirumab after prior sorafenib in patients with advanced hepatocellular carcinoma and elevated alpha-fetoprotein: Japanese subgroup analysis of the REACH-2 trial. J Gastroenterol, 55:627-639, 2020
12. Sakamoto S, Komatsu T, Watanabe R, Zhang Y, Inoue T, Kawaguchi M, Nakagawa H, Ueno T, Okusaka T, Honda K, Noji H, Urano Y. Multiplexed single-molecule enzyme activity analysis for counting disease-related proteins in biological samples. Sci Adv, 6:eaay0888, 2020
13. Athauda A, Fong C, Lau DK, Javle M, Abou-Alfa GK, Morizane C, Steward K, Chau I. Broadening the therapeutic horizon of advanced biliary tract cancer through molecular characterisation. Cancer Treat Rev, 86:101998, 2020
14. Morizane C, Okusaka T, Mizusawa J, Katayama H, Ueno M, Ikeda M, Ozaka M, Okano N, Sugimori K, Fukutomi A, Hara H, Mizuno N, Yanagimoto H, Wada K, Tobimatsu K, Yane K, Nakamori S, Yamaguchi H, Asagi A, Yukisawa S, Kojima Y, Kawabe K, Kawamoto Y, Sugimoto R, Iwai T, Nakamura K, Miyakawa H, Yamashita T, Hosokawa A, Ioka T, Kato N, Shioji K, Shimizu K, Nakagohri T, Kamata K, Ishii H, Furuse J. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol, 30:1950-1958, 2019
15. Sakamoto Y, Yamagishi S, Okusaka T, Ojima H. Synergistic and Pharmacotherapeutic Effects of Gemcitabine and Cisplatin Combined Administration on Biliary Tract Cancer Cell Lines. Cells, 8:pii: E1026, 2019
16. Komiyama S, Hijioka S, Okusaka T. Successful case of cholangioscope-assisted extraction of a radiolucent intrahepatic bile duct stent. Dig Endosc, 31:e66-e67, 2019
17. Mori M, Shimizu C, Ogawa A, Okusaka T, Yoshida S, Morita T. What determines the timing of discussions on forgoing anticancer treatment? A national survey of medical oncologists. Support Care Cancer, 27:1375-1382, 2019
18. Doi T, Aramaki T, Yasui H, Muro K, Ikeda M, Okusaka T, Inaba Y, Nakai K, Ikezawa H, Nakajima R. A phase I study of ontuxizumab, a humanized monoclonal antibody targeting endosialin, in Japanese patients with solid tumors. Invest New Drugs, 37:1061-1074, 2019
19. Sonbol MB, Ahn DH, Goldstein D, Okusaka T, Tabernero J, Macarulla T, Reni M, Li CP, O'Neil B, Van Cutsem E, Bekaii-Saab T. CanStem111P trial: a Phase III study of napabucasin plus nab-paclitaxel with gemcitabine. Future Oncol, 15:1295-1302, 2019
20. Ohmoto A, Suzuki M, Takai E, Rokutan H, Fujiwara Y, Morizane C, Yanagihara K, Shibata T, Yachida S. Correction: Establishment of preclinical chemotherapy models for gastroenteropancreatic neuroendocrine carcinoma. Oncotarget, 10:5494, 2019
21. Kashihara T, Murakami N, Iizumi S, Sakamoto Y, Nakamura S, Iijima K, Takahashi K, Inaba K, Okuma K, Igaki H, Nakayama Y, Okusaka T, Uno T, Itami J. Hemorrhage from Ascending Colon and Gluteal Muscle Associated with Sorafenib and Radiation Therapy: Radiation Dose Distribution Corresponded with Colonoscopy Findings and Computed Tomography Images. Pract Radiat Oncol, 9:214-219, 2019
22. Sunami K, Ichikawa H, Kubo T, Kato M, Fujiwara Y, Shimomura A, Koyama T, Kakishima H, Kitami M, Matsushita H, Furukawa E, Narushima D, Nagai M, Taniguchi H, Motoi N, Sekine S, Maeshima A, Mori T, Watanabe R, Yoshida M, Yoshida A, Yoshida H, Satomi K, Sukeda A, Hashimoto T, Shimizu T, Iwasa S, Yonemori K, Kato K, Morizane C, Ogawa C, Tanabe N, Sugano K, Hiraoka N, Tamura K, Yoshida T, Fujiwara Y, Ochiai A, Yamamoto N, Kohno T. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci, 110:1480-1490, 2019
23. Natsume S, Shimizu Y, Senda Y, Hijioka S, Matsuo K, Ito S, Komori K, Abe T, Hara K. Conversion surgery only for highly selected patients with unresectable pancreatic cancer: a satisfactory outcome in exchange for a lower resection rate. Surg Today, 49:670-677, 2019
24. Tamura K, Umemura Y, Hijioka S, Date K, Maehara N. Asymptomatic malignant melanoma of the gallbladder with multiple brain metastases diagnosed with endoscopic ultrasound-guided fine-needle aspiration cytology. Clin J Gastroenterol, 12:490-494, 2019
25. Ito T, Tori M, Hashigaki S, Kimura N, Sato K, Ohki E, Sawaki A, Okusaka T. Efficacy and safety of sunitinib in Japanese patients with progressive, advanced/metastatic, well-differentiated, unresectable pancreatic neuroendocrine tumors: final analyses from a Phase II study. Jpn J Clin Oncol, 49:354-360, 2019
26. Ito T, Okusaka T, Nishida T, Yamao K, Igarashi H, Morizane C, Kondo S, Mizuno N, Hara K, Sawaki A, Hashigaki S, Kimura N, Murakami M, Ohki E, Chao RC, Imamura M. Correction to: Phase II study of sunitinib in Japanese patients with unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumor. Invest New Drugs, 37:591, 2019
27. Koyama T, Kondo S, Shimizu T, Fujiwara Y, Morizane C, Sakamoto Y, Okusaka T, Yamamoto N. Impact of Hepatitis Virus on the Feasibility and Efficacy of Anticancer Agents in Patients With Hepatocellular Carcinoma in Phase I Clinical Trials. Front Oncol, 9:301, 2019
28. Yoshida T, Hijioka S, Hosoda W, Ueno M, Furukawa M, Kobayashi N, Ikeda M, Ito T, Kodama Y, Morizane C, Notohara K, Taguchi H, Kitano M, Yane K, Tsuchiya Y, Komoto I, Tanaka H, Tsuji A, Hashigo S, Mine T, Kanno A, Murohisa G, Miyabe K, Takagi T, Matayoshi N, Sakaguchi M, Ishii H, Kojima Y, Matsuo K, Yoshitomi H, Nakamori S, Yanagimoto H, Yatabe Y, Furuse J, Mizuno N. Surgery for Pancreatic Neuroendocrine Tumor G3 and Carcinoma G3 Should be Considered Separately. Ann Surg Oncol, 26:1385-1393, 2019
29. Matsubayashi H, Takaori K, Morizane C, Kiyozumi Y. Familial Pancreatic Cancer and Surveillance of High-Risk Individuals. Gut Liver, 13:498-505, 2019
30. Iwaya H, Hijioka S, Mizuno N, Kuwahara T, Okuno N, Tajika M, Tanaka T, Ishihara M, Hirayama Y, Onishi S, Ito A, Kuraoka N, Matsumoto S, Polmanee P, Shimizu Y, Yatabe Y, Niwa Y, Tamada K, Ido A, Hara K. Usefulness of septal thickness measurement on endoscopic ultrasound as a predictor of malignancy of branched-duct and mixed-type intraductal papillary mucinous neoplasm of the pancreas. Dig Endosc, 31:672-681, 2019
31. Hidaka H, Izumi N, Aramaki T, Ikeda M, Inaba Y, Imanaka K, Okusaka T, Kanazawa S, Kaneko S, Kora S, Saito H, Furuse J, Matsui O, Yamashita T, Yokosuka O, Morita S, Arioka H, Kudo M, Arai Y. Subgroup analysis of efficacy and safety of orantinib in combination with TACE in Japanese HCC patients in a randomized phase III trial (ORIENTAL). Med Oncol, 36:52, 2019
32. Maehara K, Hijioka S, Wu SYS, Ohba A, Sakamoto Y, Okusaka T, Saito Y. Re-intervention for recurrent biliary obstruction after endoscopic ultrasound hepaticogastrostomy with partially covered self-expandable metal stent. Endoscopy, 51:E297-E298, 2019
33. Iizumi S, Kuchiba A, Okusaka T, Ikeda M, Sakamoto Y, Kondo S, Morizane C, Ueno H, Osame K, Mitsunaga S, Ohno I, Imaoka H, Hashimoto Y, Takahashi H, Sasaki M, Ohashi K. Impact of the Duration of Diabetes Mellitus on the Outcome of Metastatic Pancreatic Cancer Treated with Gemcitabine: A Retrospective Study. Intern Med, 58:2435-2441, 2019
34. Ueno M, Ikeda M, Morizane C, Kobayashi S, Ohno I, Kondo S, Okano N, Kimura K, Asada S, Namba Y, Okusaka T, Furuse J. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol, 4:611-621, 2019
35. Yamazaki K, Doi T, Ikeda M, Okusaka T, Schueler A, Watanabe M, Ohtsu A. Phase I trial of pimasertib monotherapy in Japanese patients with solid tumors and those with hepatocellular carcinoma. Cancer Chemother Pharmacol, 84:1027-1037, 2019
36. Udagawa C, Horinouchi H, Shiraishi K, Kohno T, Okusaka T, Ueno H, Tamura K, Ohe Y, Zembutsu H. Whole genome sequencing to identify predictive markers for the risk of drug-induced interstitial lung disease. PLoS One, 14:e0223371, 2019
37. Terashima T, Umemoto K, Takahashi H, Hosoi H, Takai E, Kondo S, Sakamoto Y, Mitsunaga S, Ohno I, Hashimoto Y, Sasaki M, Ikeda M, Shimada K, Kaneko S, Yachida S, Sugano K, Okusaka T, Morizane C. Germline mutations in cancer-predisposition genes in patients with biliary tract cancer. Oncotarget, 10:5949-5957, 2019
38. Hattori M, Hagiwara S, Kotani H, Tatematsu M, Tachi M, Hijioka S, Shimizu J, Andoh M, Mizuno Y, Sawaki M, Yoshimura A, Gondo N, Adachi Y, Yoshimura K, Iwata H. A single-arm, phase 2 study of steroid-containing mouthwash for the prevention of everolimus-associated stomatitis in multiple tumor types. Int J Clin Oncol, 24:1320-1327, 2019
39. Bhatia V, Tajika M, Hijioka S. Radial-scanning flexible EUS of the anorectum and pelvis. Endosc Ultrasound, 8:288-297, 2019
40. Springer S, Masica DL, Dal Molin M, Douville C, Thoburn CJ, Afsari B, Li L, Cohen JD, Thompson E, Allen PJ, Klimstra DS, Schattner MA, Schmidt CM, Yip-Schneider M, Simpson RE, Fernandez-Del Castillo C, Mino-Kenudson M, Brugge W, Brand RE, Singhi AD, Scarpa A, Lawlor R, Salvia R, Zamboni G, Hong SM, Hwang DW, Jang JY, Kwon W, Swan N, Geoghegan J, Falconi M, Crippa S, Doglioni C, Paulino J, Schulick RD, Edil BH, Park W, Yachida S, Hijioka S, van Hooft J, He J, Weiss MJ, Burkhart R, Makary M, Canto MI, Goggins MG, Ptak J, Dobbyn L, Schaefer J, Sillman N, Popoli M, Klein AP, Tomasetti C, Karchin R, Papadopoulos N, Kinzler KW, Vogelstein B, Wolfgang CL, Hruban RH, Lennon AM. A multimodality test to guide the management of patients with a pancreatic cyst. Sci Transl Med, 11:pii: eaav4772, 2019
41. Ueno M, Morizane C, Ikeda M, Okusaka T, Ishii H, Furuse J. A review of changes to and clinical implications of the eighth TNM classification of hepatobiliary and pancreatic cancers. Jpn J Clin Oncol, 49:1073-1082, 2019
42. Kudo M, Ueshima K, Chiba Y, Ogasawara S, Obi S, Izumi N, Aikata H, Nagano H, Hatano E, Sasaki Y, Hino K, Kumada T, Yamamoto K, Imai Y, Iwadou S, Ogawa C, Okusaka T, Kanai F, Arai Y. Objective Response by mRECIST Is an Independent Prognostic Factor for Overall Survival in Hepatocellular Carcinoma Treated with Sorafenib in the SILIUS Trial. Liver Cancer, 8:505-519, 2019
Book
1. Hijioka S, Hara K, Mizuno N, Kuwahara T, Okuno N. EUS-BD and EUS-GBD. In: Mine T, Fujita R (eds), Advanced Therapeutic Endscopy for Pancreatico-Biliary Disease, Tokyo, Springer Japan, 2019
2. Hijioka S, Hara K, Mizuno N, Kuwahara T, Okuno N. EUS-BD and EUS-GBD. In: Mine T, Fujita R (eds), Advanced Therapeutic Endscopy for Pancreatico-Biliary Disease, Tokyo, Springer Japan, 2019
