Annual Report 2019
Department of Urology
Yoshiyuki Matsui, Motokiyo Komiyama, Yasuo Shinoda, Aiko Maejima, Hiroyuki Fujimoto
Introduction
In the Department of Urology, all urogenital malignant diseases, including adrenal cancer, kidney cancer, urothelial cancer, prostate cancer, testicular germ cell tumors, and retroperitoneal sarcoma, are the subject of diagnosis and treatment with comprehensive approaches, including radical surgery, irradiation, and chemotherapy
The Team and What We Do
The urology team consists of five staff physicians and three to four residents. In addition, with the participation of a radiation oncologist or interventional radiologist, multi-disciplinary treatments for advanced urogenital malignant diseases are performed. Every morning ward round by all staff starts at 7:30 a.m., and a weekly clinical conference is held on Monday evenings. Annual statistics of major treatment are summarized in Table 1
Major urological malignant diseases are treated according to the following strategies:
1) Renal cell carcinoma: M0, partial or radical nephrectomy (mainly laparoscopic surgery); M1: systemic treatment with targeted therapy with and/or immune checkpoint inhibitor (ICI) with or without palliative nephrectomy and metastasectomy. A selected small size (less than 3cm) tumor: cryotherapy.
2) Bladder cancer. Ta, T1, Carcinoma in situ (Cis); transurethral resection (TUR), often combined with preoperative or postoperative BCG instillation. T2-T4, radical cystectomy with neoadjuvant chemotherapy by an M-VAC/GC regimen. N+; systemic chemotherapy plus radiation or consolidation surgery; sometimes urinary diversion alone. M+; chemotherapy with an M-VAC/GC or ICI regimen.
3) Prostate cancer. Organ-confined disease, active surveillance, robotic-assisted radical prostatectomy (RARP), irradiation, or endocrine therapy. Specimen-confined disease, extended radical prostatectomy without neoadjuvant endocrine therapy, radiation therapy with endocrine therapy, or endocrine therapy alone. For high risk locally advanced prostate cancer, extended pelvic lymph node dissection is performed. N1; radiation therapy with endocrine therapy. M1; endocrine therapy and palliative radiation if necessary. For castration refractory disease, androgen receptor axis target therapy (ARAT), docetaxel or cabazitaxel chemotherapy and Ra223 are indicated.
4) Testicular germ cell tumor (GCT): Stage I, high inguinal orchiectomy and careful follow-up or adjuvant chemotherapy. Stage II or higher, EP or BEP systemic chemotherapy as the first line. In non-seminoma GCT cases, a salvage operation is performed after induction chemotherapy. In seminoma cases, careful observation rather than surgery is selected if tumor has shrunk to less than 3 cm.
Table 1. Patients statistics: Major treatment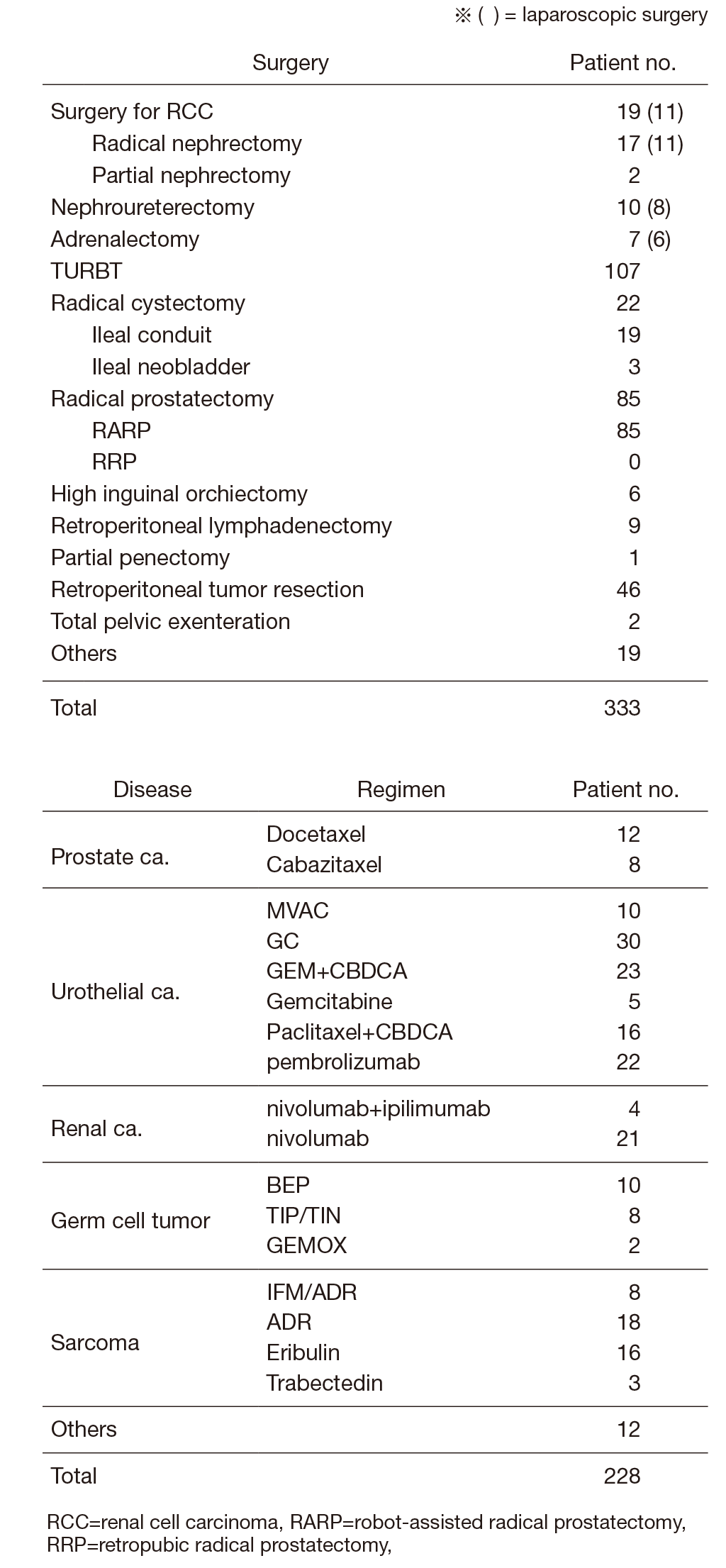

Research activities
We are constantly seeking ways to improve the treatment for malignant urological tumors.
1) Urothelial cancer: The effectiveness of a phase III study to confirm the efficacy of pirarubicin in the prevention of bladder recurrence after radical nephroureterectomy for upper tract urothelial carcinoma (JCOG1403) is ongoing. As a second or third-line chemotherapy, a weekly CBDCA + PTX regimen has been adapted.
2) Prostate cancer: A robotic operative method to achieve a complete surgical margin (extended radical prostatectomy) has been established. The significance of lymph node dissection in high risk prostate cancer has been examined in the phase II study: ICG navigated pelvic LN dissection for robotic assisted laparoscopic prostatectomy
3) Testicular GCTs: Advanced and/or refractory cases: A so-called “desperate operation”, which was designed for patients whose tumor markers did not normalize after induction chemotherapy, has been shown to be both efficacious and clinically significant. For CDDP-refractory germ cell tumors, a second line TIP/TIN, third line IrNd, GEMOX regimen has been adapted.
Clinical trials
We are actively involved in the following mainly ongoing protocol studies:
1) A phase III study: A single early intravesical instillation of pirarubicin in the prevention of bladder recurrence after radical nephroureterectomy for upper tract urothelial carcinoma (JCOG1403)
2) A Phase III, Randomized, Open-Label, Controlled, Multi-Center, Global Study of First-Line Durvalumab in Combination With Standard of Care Chemotherapy and Durvalumab in Combination With Tremelimumab and Standard of Care Chemotherapy Versus Standard of Care Chemotherapy Alone in Patients With Unresectable Locally Advanced or Metastatic Urothelial Cancer
3) A Phase II, Open-Label, Randomized, Multicenter Study to Evaluate the Efficacy and Safety of Pemigatinib Plus Pembrolizumab Versus Pemigatinib Alone Versus Standard of Care as First-Line Treatment for Metastatic or Unresectable Urothelial Carcinoma in Cisplatin-Ineligible Participants Whose Tumors Express FGFR3 Mutation or Rearrangement (FIGHT-205)
4) A phase II study: ICG navigated pelvic LN dissection for robotic assisted laparoscopic prostatectomy
Education
Skill up seminar in Urological surgery (2019/11/9)
Future prospects
To establish the standard protocol of multimodality treatment for complicated urological malignancy or retroperitoneal sarcoma
To minimize invasiveness of treatment by introduction of robotic or laparoscopic surgery
List of papers published in 2019
Journal
1. Hirata M, Asano N, Katayama K, Yoshida A, Tsuda Y, Sekimizu M, Mitani S, Kobayashi E, Komiyama M, Fujimoto H, Goto T, Iwamoto Y, Naka N, Iwata S, Nishida Y, Hiruma T, Hiraga H, Kawano H, Motoi T, Oda Y, Matsubara D, Fujita M, Shibata T, Nakagawa H, Nakayama R, Kondo T, Imoto S, Miyano S, Kawai A, Yamaguchi R, Ichikawa H, Matsuda K. Publisher Correction: Integrated exome and RNA sequencing of dedifferentiated liposarcoma. Nat Commun, 11:1024, 2020
2. Yoshida A, Arai Y, Hama N, Chikuta H, Bando Y, Nakano S, Kobayashi E, Shibahara J, Fukuhara H, Komiyama M, Watanabe SI, Tamura K, Kawai A, Shibata T. Expanding the clinicopathologic and molecular spectrum of BCOR-associated sarcomas in adults. Histopathology, 76:509-520, 2020
3. Urabe F, Matsuzaki J, Yamamoto Y, Kimura T, Hara T, Ichikawa M, Takizawa S, Aoki Y, Niida S, Sakamoto H, Kato K, Egawa S, Fujimoto H, Ochiya T. Large-scale Circulating microRNA Profiling for the Liquid Biopsy of Prostate Cancer. Clin Cancer Res, 25:3016-3025, 2019
4. Kume H, Homma Y, Shinohara N, Obara W, Kondo T, Kimura G, Fujimoto H, Nonomura N, Hongo F, Sugiyama T, Takahashi M, Kanayama HO, Fukumori T, Eto M. Perinephric invasion as a prognostic factor in non-metastatic renal cell carcinoma: analysis of a nation-wide registry program. Jpn J Clin Oncol, 49:772-779, 2019
5. Kobayashi E, Naito Y, Asano N, Maejima A, Endo M, Takahashi S, Megumi Y, Kawai A. Interim results of a real-world observational study of eribulin in soft tissue sarcoma including rare subtypes. Jpn J Clin Oncol, 49:938-946, 2019
6. Raven PA, Lysakowski S, Tan Z, D'Costa NM, Moskalev I, Frees S, Struss W, Matsui Y, Narita S, Buttyan R, Chavez-Munoz C, So AI. Inhibition of GLI2 with antisense-oligonucleotides: A potential therapy for the treatment of bladder cancer. J Cell Physiol, 234:20634-20647, 2019
7. Nakamura N, Maruyama D, Maeshima AM, Saito Y, Fujino T, Ito Y, Hatta S, Makita S, Fukuhara S, Munakata W, Suzuki T, Fujimoto H, Izutsu K. Multiple myeloma with IGH-FGFR3 rearrangement progressing as testicular plasmacytoma during carfilzomib treatment. Ann Hematol, 98:2463-2465, 2019
8. Kuroiwa K, Inokuchi J, Nishiyama H, Kojima T, Kakehi Y, Sugimoto M, Tanigawa T, Fujimoto H, Gotoh M, Masumori N, Ogawa O, Eto M, Ohyama C, Yokomizo A, Matsuyama H, Ichikawa T, Mizusawa J, Eba J, Naito S. Impact of Previous, Simultaneous or Subsequent Bladder Cancer on Prognosis after Radical Nephroureterectomy for Upper Urinary Tract Urothelial Carcinoma. J Urol, 202:1127-1135, 2019
9. Tsumura K, Arai E, Tian Y, Shibuya A, Nishihara H, Yotani T, Yamada Y, Takahashi Y, Maeshima AM, Fujimoto H, Nakagawa T, Kume H, Homma Y, Yoshida T, Kanai Y. Establishment of permutation for cancer risk estimation in the urothelium based on genome-wide DNA methylation analysis. Carcinogenesis, 40:1308-1319, 2019
10. Hirata M, Asano N, Katayama K, Yoshida A, Tsuda Y, Sekimizu M, Mitani S, Kobayashi E, Komiyama M, Fujimoto H, Goto T, Iwamoto Y, Naka N, Iwata S, Nishida Y, Hiruma T, Hiraga H, Kawano H, Motoi T, Oda Y, Matsubara D, Fujita M, Shibata T, Nakagawa H, Nakayama R, Kondo T, Imoto S, Miyano S, Kawai A, Yamaguchi R, Ichikawa H, Matsuda K. Integrated exome and RNA sequencing of dedifferentiated liposarcoma. Nat Commun, 10:5683, 2019
