Annual Report 2019
Innovation Center for Supportive, Palliative and Psychosocial Care
Yosuke Uchitomi, Takuhiro Yamaguchi, Akiko Hanai, Ayumi Okizaki, Maki Minemura, Asami Satake, Miyuki Kanamaru, Yutaka Matsuoka, Sadamoto Zenda, Eriko Satomi, Masashi Kato, Maiko Fujimori, Ayako Sato, Masako Okamura, Miyuki Odawara, Tempei Miyaji
Introduction
We have built J-SUPPORT (Japan Supportive, Palliative and Psychosocial Oncology Group) as an open hub for multi-institutional collaborative clinical research for supportive, palliative and psychosocial care and started research management throughout Japan. (Figure 1).
Figure 1. Organization of J-SUPPORT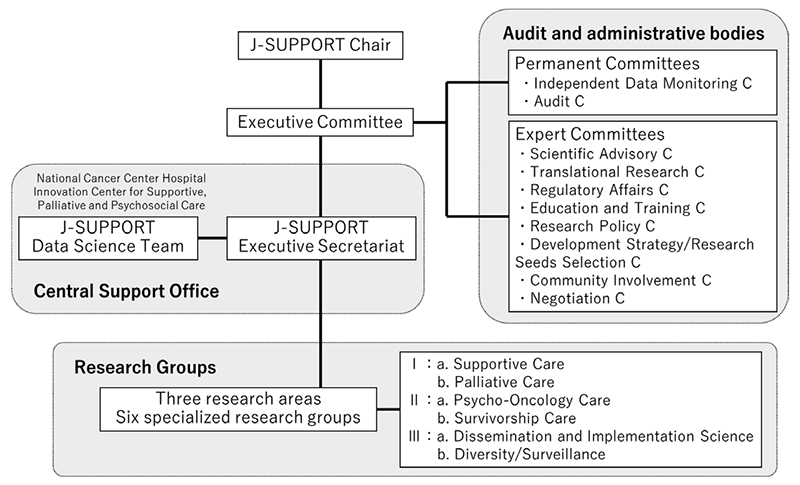

The Team and What We Do
We cooperate in providing consultation services and expert advice on clinical research design and statistical analysis to investigators as they launch new research projects in the field of supportive, palliative and psychosocial care. This service includes face-to-face clinical design and biostatistics consultation, and we coordinate collaborative studies with other study groups or institutions. We also conduct a protocol review committee and educational seminar.
Research activities
In FY 2019, 33 research consultations were conducted, with continued support for 17, and a total of 12 clinical trials approved. Four of the interventional trials were closed for enrollment, one was discontinued, three were in enrollment, one was in preparation, and two of the other clinical trials were closed and one was in analysis. The total number of annual case registrations in FY 2019 was 573. Two main articles of the approval trial were published in international journals. Furthermore, the new anti-emetic study J-SUPPORT 1604 received the Best of ASCO and Best Poster Award of ESMO. The Supportive and Palliative Care Research Policy was released by J-SUPPORT with help from the related oncology societies.
Clinical trials
- J-SUPPORT1601
Explicit prognostic disclosure to Asian women with breast cancer: A randomized, scripted video-vignette study - J-SUPPORT1602
Topical Steroid versus placebo for the prevention of radiation dermatitis in head and neck cancer patients receiving chemoradiotherapy: a phase III, randomized, double-blinded trial - J-SUPPORT1603
Nurse-led, screening-triggered early specialized palliative care intervention program for advanced lung cancer patients: a randomized controlled trial - J-SUPPORT1604
A randomized, double-blind, placebo-controlled phase III trial evaluating olanzapine 5mg combined with standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy - J-SUPPORT1605
Yokukansan for perioperative psychiatric symptoms in cancer patients undergoing high invasive surgery: a randomized, double-blind, placebo-controlled trial - J-SUPPORT1701
Efficacy of hydrocolloid dressing for prophylactic use of hand-foot skin reaction induced by multi targeted kinase inhibitors: a phase III self-controlled study - J-SUPPORT1702
Quality of palliative care for end-of-life cancer patients and retrospective cohort study of unsolved clinical question of palliative care from administrative claims data - J-SUPPORT1703
Smartphone behavioral activation and problem-solving therapy for fear of recurrence among breast cancer patients: A randomized control trial - J-SUPPORT1704
Smartphone behavioral activation and problem-solving therapy for fear of recurrence among breast cancer patients: A randomized control trial - J-SUPPORT1901
A randomized Controlled trial of the Case management to Encourage participation in cancer Screening for people with Schizophrenia in psychiatric outpatient clinics - J-SUPPORT1902
Suicide, other externally caused injuries, and cardiovascular death following a cancer diagnosis: a nationwide population-based study in Japan - J-SUPPORT1903
The single-armed confirmatory trial for immediate effectivity and safety of palliative arterial embolization for painful bone metastases
Education
In FY 2019, the D&I Science Society (Dissemination and Implementation Science Society) 2nd and 3rd Scientific Meetings (95 and 93 participants, respectively), the 4th Japan Society for Cancer Supportive Care JASCC & J-SUPPORT Joint Workshop and JORTC x J-SUPPORT Joint Research Conference (50 participants each), the Japanese Society for Lipid Nutrition 1st Young Researcher Development Seminar (20 participants), the Psycho-Oncology Training Seminar (18 participants), and the 5th J-SUPPORT Seminar (34 participants) were held.
In addition, the 1st J-SUPPORT research results debriefing meeting, "Let's create a world where people can cope with cancer! -from the front lines of research on supportive, palliative and psycho-oncological care-" was held.
Future prospects
Support the conduct of J-SUPPORT-approved clinical research, facilitate the approval of new clinical trials, engage in patient and public involvement (PPI), and promote research consultation and educational activities. In addition, as the research areas are diversified, we will collaborate with other research organizations to further expand our research achievements.
List of papers published in 2019
Journal
1. Hashimoto H, Abe M, Tokuyama O, Mizutani H, Uchitomi Y, Yamaguchi T, Hoshina Y, Sakata Y, Takahashi TY, Nakashima K, Nakao M, Takei D, Zenda S, Mizukami K, Iwasa S, Sakurai M, Yamamoto N, Ohe Y. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol, 21:242-249, 2020
2. Matsuoka YJ, Okubo R, Shimizu Y, Tsuji K, Narisawa T, Sasaki J, Sasai H, Akashi-Tanaka S, Hamaguchi T, Iwasa T, Iwata S, Kato T, Kurotani K, Maruyama D, Mori A, Ogawa A, Sakurai N, Shimazu T, Shimizu C, Tabuchi T, Takahashi M, Takano T, Tatematsu N, Uchitomi Y, Watanabe C, Fukui T. Developing the structure of Japan's cancer survivorship guidelines using an expert panel and modified Delphi method. J Cancer Surviv, 14:273-283, 2020
3. Matsuda Y, Tanimukai H, Inoue S, Inada S, Sugano K, Hasuo H, Yoshimura M, Wada S, Dotani C, Adachi H, Okamoto Y, Takeuchi M, Fujisawa D, Kako J, Sasaki C, Kishi Y, Akizuki N, Inagaki M, Uchitomi Y, Matsushima E, Okuyama T. JPOS/JASCC clinical guidelines for delirium in adult cancer patients: a summary of recommendation statements. Jpn J Clin Oncol, 50:586-593, 2020
4. Yatsuoka W, Ueno T, Miyano K, Uezono Y, Enomoto A, Kaneko M, Ota S, Soga T, Sugimoto M, Ushijima T. Metabolomic profiling reveals salivary hypotaurine as a potential early detection marker for medication-related osteonecrosis of the jaw. PLoS One, 14:e0220712, 2019
5. Uzu M, Nonaka M, Miyano K, Sato H, Kurebayashi N, Yanagihara K, Sakurai T, Hisaka A, Uezono Y. A novel strategy for treatment of cancer cachexia targeting xanthine oxidase in the brain. J Pharmacol Sci, 140:109-112, 2019
6. Manabe S, Miyano K, Fujii Y, Ohshima K, Yoshida Y, Nonaka M, Uzu M, Matsuoka Y, Sato T, Uezono Y, Morimatsu H. Possible biased analgesic of hydromorphone through the G protein-over beta-arrestin-mediated pathway: cAMP, CellKey, and receptor internalization analyses. J Pharmacol Sci, 140:171-177, 2019
7. Miyano K, Shiraishi S, Minami K, Sudo Y, Suzuki M, Yokoyama T, Terawaki K, Nonaka M, Murata H, Higami Y. Carboplatin Enhances the Activity of Human Transient Receptor Potential Ankyrin 1 through the Cyclic AMP-Protein Kinase A-A-Kinase Anchoring Protein (AKAP) Pathways. Int J Mol Sci, 20:E3271, 2019
8. Wada S, Sadahiro R, Matsuoka YJ, Uchitomi Y, Yamaguchi T, Shimizu K. Yokukansan for perioperative psychiatric symptoms in cancer patients undergoing high invasive surgery. J-SUPPORT 1605 (ProD Study): study protocol for a randomized controlled trial. Trials, 20:110, 2019
9. Wada S, Inoguchi H, Sadahiro R, Matsuoka YJ, Uchitomi Y, Sato T, Shimada K, Yoshimoto S, Daiko H, Shimizu K. Preoperative Anxiety as a Predictor of Delirium in Cancer Patients: A Prospective Observational Cohort Study. World J Surg, 43:134-142, 2019
10. Higuchi Y, Fujiwara M, Nakaya N, Fujimori M, Hayashibara C, So R, Shinkawa I, Sato K, Yada Y, Kodama M, Takenaka H, Kishi Y, Kakeda K, Uchitomi Y, Yamada N, Inagaki M. Change in smoking cessation stage over 1 year in patients with schizophrenia: a follow up study in Japan. BMC Psychiatry, 19:367, 2019
11. Mori M, Fujimori M, van Vliet LM, Yamaguchi T, Shimizu C, Kinoshita T, Morishita-Kawahara M, Inoue A, Inoguchi H, Matsuoka Y, Bruera E, Morita T, Uchitomi Y. Explicit prognostic disclosure to Asian women with breast cancer: A randomized, scripted video-vignette study (J-SUPPORT1601). Cancer, 125:3320-3329, 2019
12. Okubo R, Wada S, Shimizu Y, Tsuji K, Hanai A, Imai K, Uchitomi Y, Fujiwara Y, Tsugane S, Matsuoka YJ. Expectations of and recommendations for a cancer survivorship guideline in Japan: a literature review of guidelines for cancer survivorship. Jpn J Clin Oncol, 49:812-822, 2019
13. Fujiwara M, Inagaki M, Shimazu T, Kodama M, So R, Matsushita T, Yoshimura Y, Horii S, Fujimori M, Takahashi H, Nakaya N, Kakeda K, Miyaji T, Hinotsu S, Harada K, Okada H, Uchitomi Y, Yamada N. A randomised controlled trial of a case management approach to encourage participation in colorectal cancer screening for people with schizophrenia in psychiatric outpatient clinics: study protocol for the J-SUPPORT 1901 (ACCESS) study. BMJ Open, 9:e032955, 2019
14. Harashima S, Fujimori M, Akechi T, Matsuda T, Saika K, Hasegawa T, Inoue K, Yoshiuchi K, Miyashiro I, Uchitomi Y, Matsuoka YJ. Suicide, other externally caused injuries and cardiovascular death following a cancer diagnosis: study protocol for a nationwide population-based study in Japan (J-SUPPORT 1902). BMJ Open, 9:e030681, 2019
15. Mori M, Fujimori M, Ishiki H, Nishi T, Hamano J, Otani H, Uneno Y, Oba A, Morita T, Uchitomi Y. Adding a Wider Range and “Hope for the Best, and Prepare for the Worst” Statement: Preferences of Patients with Cancer for Prognostic Communication. Oncologist, 24:e943-e952, 2019
16. Mori M, Fujimori M, Ishiki H, Nishi T, Hamano J, Otani H, Uneno Y, Oba A, Morita T, Uchitomi Y. The Effects of Adding Reassurance Statements: Cancer Patients' Preferences for Phrases in End-of-Life Discussions. J Pain Symptom Manage, 57:1121-1129, 2019
