Annual Report 2019
Clinical Research Support Office
- Clinical Research Coordinating Division
Hideki Ueno, Miki Ito, Katsuaki Imaizumi, Kikue Hasegawa, Asako Sakamoto, Emi Yasuda, Sho Murata, Mari Takahashi, Hiroko Kawaguchi, Shinobu Araki, Chiharu Nakano, Asako Kamikawa, Setsuko Kamizaki, Yoshimi Yamaguchi, Yumiko Ikuno, Kimiyo Yoshii, Katsuyuki Igarashi, Hiroko Takagi, Maki Takayasu, Aya Matsumoto, Yukako Takasaki, Kayo Sawaki, Ran Obara, Shoko Kusaka, Harue Ui, Tamami Yamano, Kayo Ohsugi, Nahoko Hiraoka, Tomomi Tsuchiya, Chie Miyano, Hatsuki Ohno, Noriko Makita, Chikano Yashiro, Eri Yanagisawa, Yukari Nishiyama, Azumi Seko, Ai Sekido, Kazumi Matsuo, Noriko Yoshida, Rie Nagao, Kumiko Hirayama, Ami Hashimoto, Chihiro Suda, Risa Takamizawa, Ritsuko Yamamoto, Kazumi Higuchi, Ai Kobayakawa, Maya Niigawa, Hiroe Takayama, Mayumi Miyamoto, Mari Kondo, Chie Motegi, Kimiko Sega, Nobuko Ushirozawa, Yoko Ebihara, Harumi Maruno, Junko Horie, Eiko Mimata, Kaori Matsushita, Yuriko Shinotsuka, Yuki Sezaki, Satoko Murakami, Nozami Iida, Chiaki Yamamoto, Ken Kato, Teiko Yamane, Keiko Wakakuwa, Keiko Shimo, Mayumi Ikeda, Yuki Murai, Satomi Nakamori, Harumi Mochizuki, Riko Tamura, Noboru Yamamoto - Research Management Division
Kenichi Nakamura, Ayaka Omine, Sachi Nishikawa, Mamiko Nakata, Rena Nakazawa, Tomomi Hata, Makiko Watanabe, Mitsumi Terada, Takako Hitomi, Laureline Gatellier, Natsuko Okita, Tamie Sukigara, Mamiko Kawasaki, Satoshi Kawashima, Hitomi Okuma, Naoko Matsui, Hitoshi Ozawa, Masahiko Ichimura, Shoko Narita, Sae Ishimaru, Yohei Otake, Hiroshi Katayama, Sawako Tomatsuri, Sachie Kawabata, Eiko Yorikane, Yoshie Shuda, Kenta Anjo, Kaori Izumino, Junko Eba, Keisuke Kanato, Tomoko Kataoka, Keita Sasaki, Ryo Shimoyama, Hideki Masai, Kaori Umegai, Taro Shibata, Aya Kuchiba, Junki Mizusawa, Gakuto Ogawa, Ryunosuke Machida, Shun Sadachi, Akihiro Hirakawa, Seiji Sasaki, Ryo Kitabayashi, Chihiro Yaita, Yuki Konda - Data Management Division
Haruhiko Fukuda, Harumi Kaba, Mikio Mori, Nobuko Okamura, Ryuji Makiuchi, Keiko Ohata, Yukari Hoshina, Tomoaki Yamada, Masahisa Kamikura, Yukari Nagasaka, Eiko Sayama, Eru Adachi, Minako Nagase, Keiko Suto
Introduction
The Clinical Research Support Office supports clinical research conducted under the leadership of investigators in the National Cancer Center Hospital (NCCH). Support activities include protocol writing, central/local data management, statistical design and analysis, in-house/on-site monitoring, audits, patient recruitment, and other coordinating jobs.
The Team and What We Do
- Clinical Research Coordinating Division
The Clinical Research Coordinating Section and the Clinical Trial Administration Section support a lot of industry-sponsored registration trials as well as physician-initiated registration-directed clinical trials. A total of 40 CRCs (clinical research coordinators), 10 CRC assistants, and 11 administration staff support these trials.
The Biobank and Translational Research Support Section has routinely obtained informed consent to participate as an NCC biobank (NCCBB) donor from patients who consult with the NCCH for the first time. CRCs in this section coordinate translational research in several ways, such as assistance for registration for clinical trials, logistics for pathological specimens, data collection for case report forms, and coordination between sections. We explained the purport of the NCCBB to 9,696 patients from Apr 2019 to Mar 2020, and received consent for blood collection and use of their surplus samples for research from 8,841 patients (91.2% consent rate). The patient load with our assistance in filling in the preliminary-diagnosis card and so on was 10,070. We also support eight biomarker studies, and the registered patients (pts) were as follows: 263 pts for MASTER KEY project, 29 pts for micro RNA project, 11 pts for GI-SCREEN_CRC study, 61 pts for MASTER KEY HEM study, 14 pts for GI-SCREEN Registry, 107 pts for GOZILA study, 32 pts for MONSTAR SCREEN study and 843 pts for CiCLE study.
- Research Management Division
The Research Management Division is in charge of central research support functions: i) International clinical trial management, ii) Investigator-initiated registration-directed trial (Chiken) management, iii) Monitoring & consultation, iv) Multi-institutional clinical trial support, v) Biostatistics, vi) Pharmaceutical affairs consultation. The division has the capability to support various types of clinical trials covering both early phase ones including first-in-human trials and late phase multi-institutional trials. For the early phase trials, the division mainly offers comprehensive study management, site visit monitoring and safety information management. One of the strengths of the division is that it can coordinate not only domestic trials but also international investigator-initiated registration-directed trials. The multi-institutional trial support function works as the Japan Clinical Oncology Group (JCOG) Operations Office which engages in protocol development, manuscript drafting, study coordination, and so forth for late phase trials.
- Data Management Division
The Data Management Division is responsible for central data management and in-house study monitoring in investigator-initiated clinical trials for cancer therapeutic development. The Data Management Section supports early phase cancer trials mainly for drug development including registration trials which are led by physicians in the NCCH. The Multi-institutional Data Management Section supports mostly late development multi-modality multi-institutional phase II or phase III trials for adult solid cancer conducted by the JCOG.
Research activities
- Research Management Division
At academic conferences, several presentations were made by section members on topics such as: i) NCCH initiatives as a Clinical Research Core Hospital; ii) Issues and solutions to facilitate clinical trials under the Clinical Trials Act; iii) Project management of investigator-initiated registration-directed trials, Advanced Medical Care studies, and patient-proposed health services.
Clinical trials
- Clinical Research Coordinating Division
The number of industry-sponsored registration trials is increasing year by year, and the division supported 446 registration-directed clinical trials including 51 investigator-initiated registration-directed trials in FY 2019 (Table 1).
Table 1. Supported Trials in Clinical Research Coordinating Division in FY 2019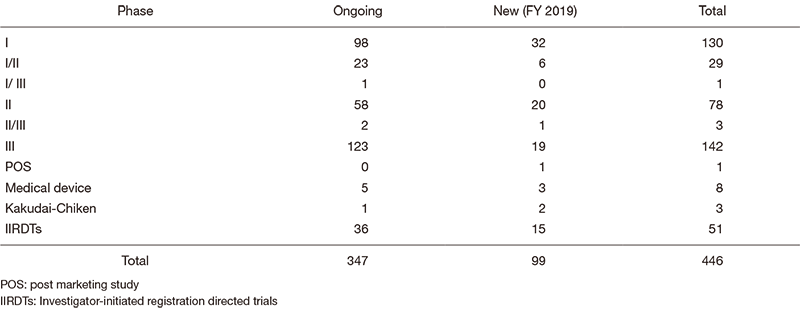

- Research Management Division
The priority area of the division is genomic medicine, rare cancer and international trials. As of the end of the fiscal year 2019, the Research Management Division supported 20 investigator-initiated registration-directed trials, ten clinical trials using the Advanced Medical Care system and two trials under patient-proposed health services. This division has been in charge of the coordinating office of rare cancer registry study with basket sub-studies (MASTER KEY Project). This division also supports an international investigator-initiated registration-directed trial (PATHWAY trial).
- Data Management Division
The Data Management Section supports 17 IND trials (9 open, 3 in preparation, 5 on follow-up) and 8 non-IND studies (5 open, 2 in preparation, 1 on follow-up). The Multi-institutional Data Management Section supports 98 non-IND trials (47 open, 18 in preparation, 33 on follow-up) as the JCOG Data Center.
Education
The staff members received not only day-to-day on-the-job training but also in-house educational seminars and the JCOG internal educational programs in order to learn clinical trial methodology, project management, monitoring, and research ethics
Future prospects
- Clinical Research Coordinating Division
The number of supported clinical trials is increasing as previously described, and the supported area covered by CRCs will be expanded to include not only registration trials but also other investigator-initiated clinical trials. Therefore, expansion of CRC staff members is highly demanded. In view of the plan for the NCCH, all members of this office will work together to contribute to reinforcing clinical research capabilities of the NCCH and to making this office a valuable unit for all members of our hospital.
The members of the Biobank and Translational Research Support Section received seminars which are related to clinical research ethics, etc. The section informs and educates investigators of the NCC about the NCC biobank and translational research periodically through the NCC University. As a future direction, the section will improve the quality of the NCC biobank’s informatics and storage of serum. The section also aims to establish a support system for higher quality and quantity.
- Research Management Division
Since the number of investigator-initiated registration-directed trials has increased, reinforcement of staff resources is urgently needed. In response to this increase, this division will reinforce the support function for various clinical trials including international clinical trials, investigator-initiated registration-directed trials, Advanced Medical Care system trials, and patient-proposed health services trials. Also, this division will establish optimal ways to cope with the newly enacted Clinical Research Act.
- Data Management Division
The Data Management Division is introducing a web-based electronic data capturing (EDC) system and is promoting standardization of all aspects of data management, such as data formats, case report forms and monitoring reports for increased data integrity, and cost effectiveness of day-to-day work.
