Annual Report 2019
Division of Carcinogenesis and Cancer Prevention
(Viral Carcinogenesis and Cancer Prevention Group)
Tohru Kiyono, Tomomi Nakahara, Takako Ishiyama, Kasumi Dendo, Katsuyuki Tanaka, Chiho Kohno, Yukimi Gotoh, Etsuko Kabasawa, Takashi Yugawa, Shin-ichi Ohno, Yuki Inagawa
Introduction
Approximately 15% of human cancers have a viral etiology. Oncogenic HPV (Human Papillomavirus) infection accounts for almost 5% of all cancers worldwide, including cervical, anal, vaginal, vulvar, penile, and head and neck cancers. The expression of viral E6 and E7 proteins is primary responsible for cancer development by inactivating the major tumor suppressors, p53 and retinoblastoma protein (pRB), respectively. The aim of the research in the Viral Carcinogenesis and Cancer Prevention Group is to elucidate the molecular mechanisms for carcinogenesis and viral persistence of HPV infection in order to develop effective anti-HPV therapy.
The Team and What We Do
To study molecular pathology of cancers with or without viral etiology, we are developing ex vivo carcinogenesis models by transducing genetic abnormalities frequently found in given cancers into their respective normal cells-of-origin. With our tissue culture models, we aim to (1) elucidate mechanisms of viral persistence of HPV infection and (2) analyze the association between driver mutations and cancer characteristics.
Research activities
1. HPV-induced carcinogenesis and its prevention
To develop new strategies for viral elimination to prevent HPV-associated cancer development, it is critical to gain insight into the molecular mechanisms of the viral genome replication. The HPV life cycle depends on the differentiation of the stratified epithelium. In basal cells, viral genomes are maintained as low copy number-episomes and expression of the viral genes is minimal. This latent state is thought to facilitate persistent infection by eluding host immune surveillance.
1-1. We demonstrated that the viral helicase, E1 is dispensable for maintaining HPV episomes in basal cells. Subsequent studies indicated that E1 expression induces activation of the NFκB pathway and NFκB functions as a negative feedback loop by facilitating phosphorylation-dependent proteasomal degradation of E1. We propose that this negative feedback loop is a key molecular mechanism for the viral persistence by strictly limiting the E1 level in basal cells (Figure 1). We are currently investigating whether the dissolution of this feedback loop leads to elimination of virus infection.
Figure 1. Concept of anti-HPV strategy by intervention in E1-NFkB negative feedback loop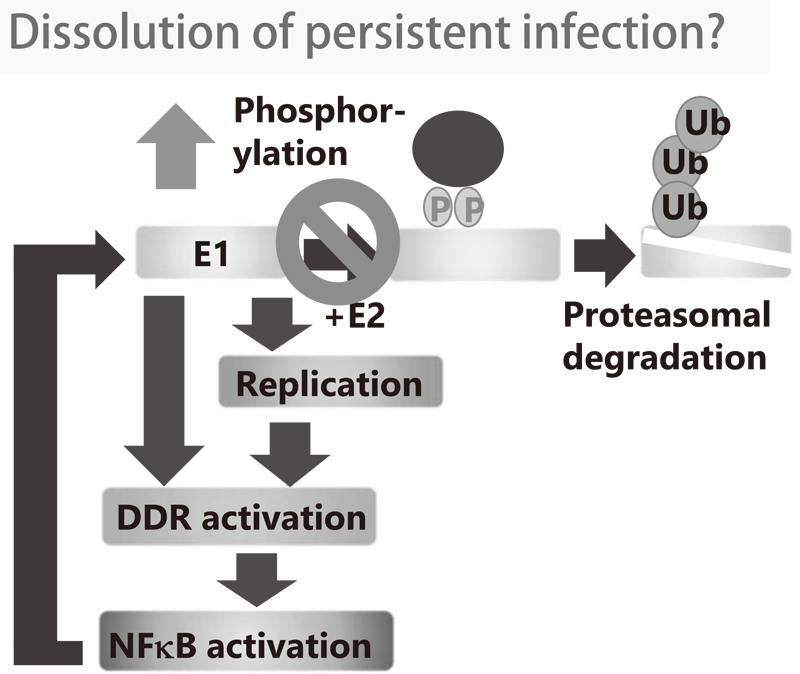

1-2. Genome editing technologies such as CRISPR/Cas9 made it possible to directly target the HPV genome. We developed an adenovirus vector expressing multiple gRNAs targeting HPV16 E6/E7 coding regions and found that it suppresses cell growth of several HPV16 positive cervical cancer cells as well as its precursor models generated in our laboratory. This vector was able to target integrated HPV genomes as well as episomal genomes (Figure 2).
Figure 2. CRISPR/Cas9 HPV targeting vector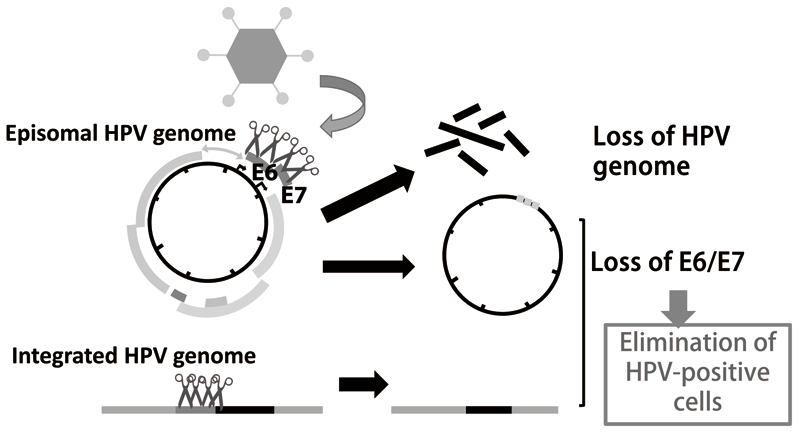

2. In vitro human carcinogenesis model
We have been studying the biological significance ofΔNp63α (hereafter just p63) expression and the molecular basis underlying acquisition of malignant traits in squamous cell carcinomas (SCCs) in cervical and head and neck cancers. p63 plays an essential role in stem cell maintenance of stratified epithelium partly through transcriptional suppression of NOTCH1. Indeed, overexpression of p63 and inactivating mutation of NOTCH1 are frequently seen in human SCCs including examples in the cervix, lung, and head and neck. Paradoxically, loss of p63 is shown to be associated with metastasis and poor prognosis in SCCs, although the underlying molecular mechanism and its functional relevance to carcinogenesis remains unclear as is the case with NOTCH1. We have demonstrated that loss of p63 induces terminal differentiation of normal keratinocytes through activation of the NOTCH-ROCK pathway. In MYC-overexpressing cells, however, knock-down of p63 conferred increased invasiveness through the NOTCH-ROCK pathway (Figure 3). MRTF-A (MKL1) as a downstream effector in the NOTCH-ROCK pathway induced keratinocyte differentiation. Our goal is to develop novel strategies for diagnosis and molecularly targeted therapy against poorly-differentiated SCCs based on its cancer biology.
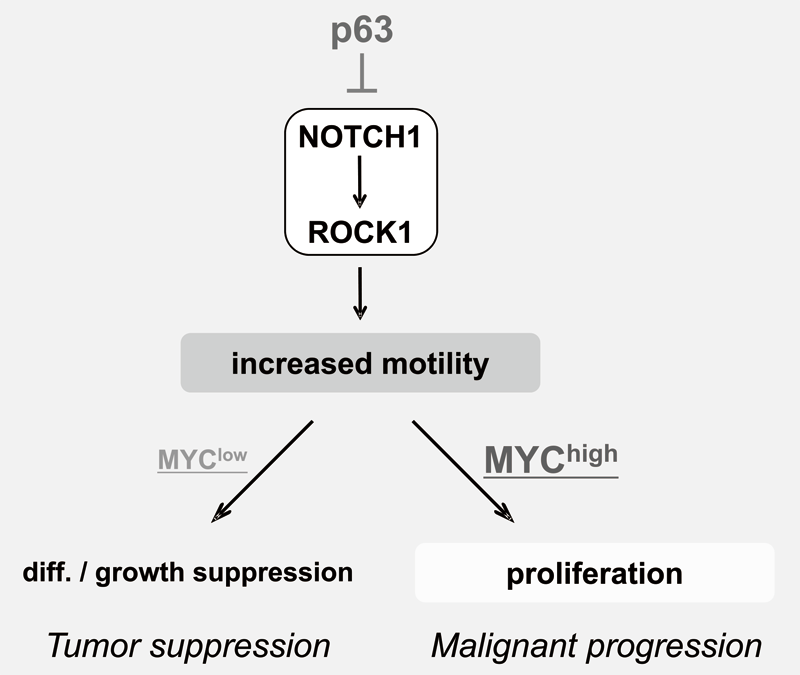
Education
A PhD student from Khon Kaen University, Thailand was trained in our laboratory as a part of her PhD program. Two Japanese graduate students completed the program and received Master degree from Kitasato University and Tokyo Medical and Dental University. A medical doctor from China continued the PhD program of Keio University, Graduate school of Medicine in our laboratory.
Future prospects
We are strongly promoting development of an HPV targeting gene therapy with CRISPR/Cas9 systems since promising results were obtained. Based on our findings of molecular mechanisms for viral persistence, we are developing a new approach for the eradication of HPV infection. The in vitro carcinogenesis model for any given cancer can be established in vitro with our techniques. These models will be useful for preclinical assessment of new cancer therapies.
List of papers published in 2019
Journal
1. Yang XY, Ozawa S, Kato Y, Maehata Y, Izukuri K, Ikoma T, Kanamori K, Akasaka T, Suzuki K, Iwabuchi H, Kurata SI, Katoh I, Sakurai T, Kiyono T, Hata RI. C-X-C Motif Chemokine Ligand 14 is a Unique Multifunctional Regulator of Tumor Progression. Int J Mol Sci, 20:2019
2. Tani T, Eitsuka T, Katayama M, Nagamine T, Nakaya Y, Suzuki H, Kiyono T, Nakagawa K, Inoue-Murayama M, Onuma M, Fukuda T. Establishment of immortalized primary cell from the critically endangered Bonin flying fox (Pteropus pselaphon). PLoS One, 14:e0221364, 2019
3. Sekine S, Kiyono T, Ryo E, Ogawa R, Wakai S, Ichikawa H, Suzuki K, Arai S, Tsuta K, Ishida M, Sasajima Y, Goshima N, Yamazaki N, Mori T. Recurrent YAP1-MAML2 and YAP1-NUTM1 fusions in poroma and porocarcinoma. J Clin Invest, 130:3827-3832, 2019
4. Naito Y, Yamamoto Y, Sakamoto N, Shimomura I, Kogure A, Kumazaki M, Yokoi A, Yashiro M, Kiyono T, Yanagihara K, Takahashi RU, Hirakawa K, Yasui W, Ochiya T. Cancer extracellular vesicles contribute to stromal heterogeneity by inducing chemokines in cancer-associated fibroblasts. Oncogene, 38:5566-5579, 2019
5. Murakami I, Egawa N, Griffin H, Yin W, Kranjec C, Nakahara T, Kiyono T, Doorbar J. Roles for E1-independent replication and E6-mediated p53 degradation during low-risk and high-risk human papillomavirus genome maintenance. PLoS Pathog, 15:e1007755, 2019
6. Katayama M, Kiyono T, Ohmaki H, Eitsuka T, Endoh D, Inoue-Murayama M, Nakajima N, Onuma M, Fukuda T. Extended proliferation of chicken- and Okinawa rail-derived fibroblasts by expression of cell cycle regulators. J Cell Physiol, 234:6709-6720, 2019
7. Katayama M, Kiyono T, Kuroda K, Ueda K, Onuma M, Shirakawa H, Fukuda T. Rat-derived feeder cells immortalized by expression of mutant CDK4, cyclin D, and telomerase can support stem cell growth. Biochim Biophys Acta Mol Cell Res, 1866:945-956, 2019
8. Hirose Y, Onuki M, Tenjimbayashi Y, Yamaguchi-Naka M, Mori S, Tasaka N, Satoh T, Morisada T, Iwata T, Kiyono T, Mimura T, Sekizawa A, Matsumoto K, Matsumoto K, Kukimoto I. Whole-Genome Analysis of Human Papillomavirus Type 16 Prevalent in Japanese Women with or without Cervical Lesions. Viruses, 11:2019
9. Ghani FI, Dendo K, Watanabe R, Yamada K, Yoshimatsu Y, Yugawa T, Nakahara T, Tanaka K, Yoshida H, Yoshida M, Ishikawa M, Goshima N, Kato T, Kiyono T. An Ex-Vivo Culture System of Ovarian Cancer Faithfully Recapitulating the Pathological Features of Primary Tumors. Cells, 8:2019
10. Hirose S, Murakami N, Takahashi K, Kuno I, Takayanagi D, Asami Y, Matsuda M, Shimada Y, Yamano S, Sunami K, Yoshida K, Honda T, Nakahara T, Watanabe T, Komatsu M, Hamamoto R, Kato MK, Matsumoto K, Okuma K, Kuroda T, Okamoto A, Itami J, Kohno T, Kato T, Shiraishi K, Yoshida H. Genomic alterations in STK11 can predict clinical outcomes in cervical cancer patients. Gynecol Oncol, 156:203-210, 2020
11. Tani T, Eitsuka T, Katayama M, Nagamine T, Nakaya Y, Suzuki H, Kiyono T, Nakagawa K, Inoue-Murayama M, Onuma M, Fukuda T. Establishment of immortalized primary cell from the critically endangered Bonin flying fox (Pteropus pselaphon). PLoS One, 14:e0221364, 2019
12. Sekine S, Kiyono T, Ryo E, Ogawa R, Wakai S, Ichikawa H, Suzuki K, Arai S, Tsuta K, Ishida M, Sasajima Y, Goshima N, Yamazaki N, Mori T. Recurrent YAP1-MAML2 and YAP1-NUTM1 fusions in poroma and porocarcinoma. J Clin Invest, 129:3827-3832, 2019
13. Naito Y, Yamamoto Y, Sakamoto N, Shimomura I, Kogure A, Kumazaki M, Yokoi A, Yashiro M, Kiyono T, Yanagihara K, Takahashi RU, Hirakawa K, Yasui W, Ochiya T. Cancer extracellular vesicles contribute to stromal heterogeneity by inducing chemokines in cancer-associated fibroblasts. Oncogene, 38:5566-5579, 2019
14. Katayama M, Kiyono T, Ohmaki H, Eitsuka T, Endoh D, Inoue-Murayama M, Nakajima N, Onuma M, Fukuda T. Extended proliferation of chicken- and Okinawa rail-derived fibroblasts by expression of cell cycle regulators. J Cell Physiol, 234:6709-6720, 2019
15. Katayama M, Kiyono T, Kuroda K, Ueda K, Onuma M, Shirakawa H, Fukuda T. Rat-derived feeder cells immortalized by expression of mutant CDK4, cyclin D, and telomerase can support stem cell growth. Biochim Biophys Acta Mol Cell Res, 1866:945-956, 2019
16. Ghani FI, Dendo K, Watanabe R, Yamada K, Yoshimatsu Y, Yugawa T, Nakahara T, Tanaka K, Yoshida H, Yoshida M, Ishikawa M, Goshima N, Kato T, Kiyono T. An Ex-Vivo Culture System of Ovarian Cancer Faithfully Recapitulating the Pathological Features of Primary Tumors. Cells, 8:644. doi: 10.3390/cells8070644, 2019
17. Fukuda T, Gouko R, Eitsuka T, Suzuki R, Takahashi K, Nakagawa K, Sugano E, Tomita H, Kiyono T. Human-Derived Corneal Epithelial Cells Expressing Cell Cycle Regulators as a New Resource for in vitro Ocular Toxicity Testing. Front Genet, 10:587, 2019
18. Hirose Y, Onuki M, Tenjimbayashi Y, Yamaguchi-Naka M, Mori S, Tasaka N, Satoh T, Morisada T, Iwata T, Kiyono T, Mimura T, Sekizawa A, Matsumoto K, Kukimoto I. Whole-Genome Analysis of Human Papillomavirus Type 16 Prevalent in Japanese Women with or without Cervical Lesions. Viruses, 11:350. doi: 10.3390/v11040350, 2019
19. Inoue M, Kajiwara K, Yamaguchi A, Kiyono T, Samura O, Akutsu H, Sago H, Okamoto A, Umezawa A. Autonomous trisomic rescue of Down syndrome cells. Lab Invest, 99:885-897, 2019
20. Murakami I, Egawa N, Griffin H, Yin W, Kranjec C, Nakahara T, Kiyono T, Doorbar J. Roles for E1-independent replication and E6-mediated p53 degradation during low-risk and high-risk human papillomavirus genome maintenance. PLoS Pathog, 15:e1007755, 2019
21. Yang XY, Ozawa S, Kato Y, Maehata Y, Izukuri K, Ikoma T, Kanamori K, Akasaka T, Suzuki K, Iwabuchi H, Kurata SI, Katoh I, Sakurai T, Kiyono T, Hata RI. C-X-C Motif Chemokine Ligand 14 is a Unique Multifunctional Regulator of Tumor Progression. Int J Mol Sci, 20:1872. doi: 10.3390/ijms20081872, 2019
22. Omori H, Nishio M, Masuda M, Miyachi Y, Ueda F, Nakano T, Sato K, Mimori K, Taguchi K, Hikasa H, Nishina H, Tashiro H, Kiyono T, Mak TW, Nakao K, Nakagawa T, Maehama T, Suzuki A. YAP1 is a potent driver of the onset and progression of oral squamous cell carcinoma. Sci Adv, 6:eaay3324, 2020
23. Morris TA, Naik J, Fibben KS, Kong X, Kiyono T, Yokomori K, Grosberg A. Striated myocyte structural integrity: Automated analysis of sarcomeric z-discs. PLoS Comput Biol, 16:e1007676, 2020
24. Orimoto A, Kyakumoto S, Eitsuka T, Nakagawa K, Kiyono T, Fukuda T. Efficient immortalization of human dental pulp stem cells with expression of cell cycle regulators with the intact chromosomal condition. PLoS One, 15:e0229996, 2020
25. Heawchaiyaphum C, Iizasa H, Ekalaksananan T, Burassakarn A, Kiyono T, Kanehiro Y, Yoshiyama H, Pientong C. Epstein-Barr Virus Infection of Oral Squamous Cells. Microorganisms, 8:419. doi: 10.3390/microorganisms8030419, 2020
