Annual Report 2019
Division of Cancer Immunology
Hiroyoshi Nishikawa, Yuka Maeda, Keisuke Watanabe, Hitomi Nishinakamura,Kentaro Shinohara, Hiroaki Hiramatsu,Maki Sugaya, Kota Itahashi, Daichi Matsumoto, Ryuto Tsuchiya, Sayoko Shinya, Tadaaki Ioroi, Kana Kusaba, Ai Sasaki, Etsuko Tanji, Priya Saju, Yuki Ishige, Nanae Shimoyama, Mizuki Marumo
Introduction
Cancer immunotherapies, particularly immune checkpoint inhibitors (ICIs) such as anti-CTLA-4 mAb and anti-PD-(L)1 mAb, are becoming important strategies to treat cancer. However, the clinical effects of ICIs are observed in limited patients because cancer cells establish a complex immune suppressive mechanism that inhibits anti-tumor immune responses. To reveal the mechanisms is an urgent requirement for the development of effective cancer immunotherapy (Figure 1). Our aims are clarifying the detailed mechanism of immune suppression in a tumor microenvironment by immunologic and genetic approaches and identifying novel approaches to control anti-tumor immune responses.
Figure 1. Investigation of dynamic immune state in cancer patients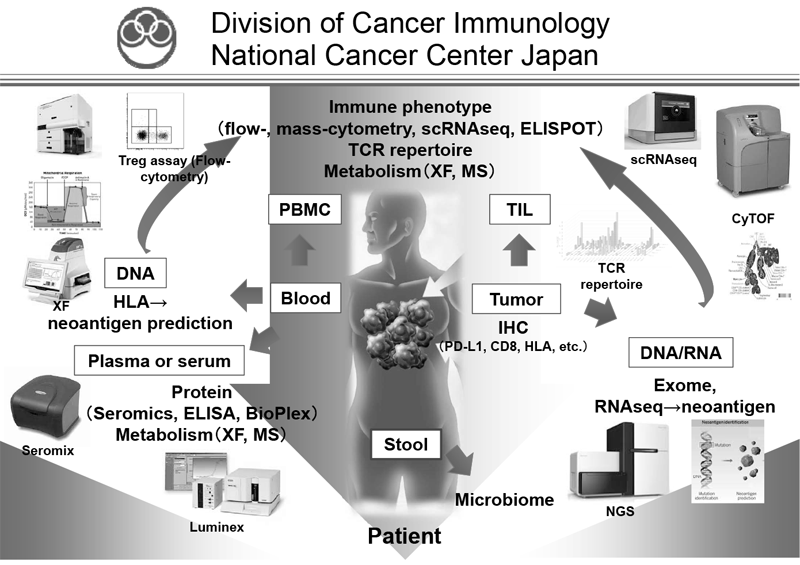

Figure 2. Development of novel CAR-T therapies and plan to perform clinical trial 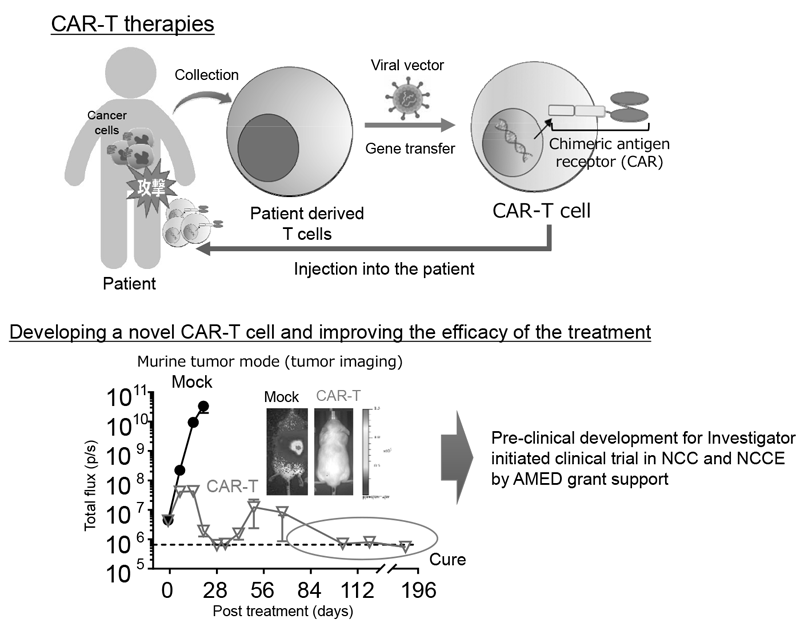

The Team and What We Do
Our division focused on elucidating the detailed mechanisms involved in immune suppression or tumor immune evasion in various types of cancers. We have collected tissues and peripheral blood pre- and post-treatment with immune checkpoint inhibitors from many cancer patients. To comprehensively explore immune responses, molecular markers and gene expression profiles of TILs and PBMCs were examined.
Research activities
We established strategies to investigate multiple parameters at a single-cell level using flow cytometry and mass cytometry. Lymphocytes are known to change their metabolism between glycolysis and oxidative phosphorylation according to their activity states and cell types such as effector and regulatory T cells. We then interrogated the control mechanism of metabolic shift of lymphocytes in immune suppression or immune evasion in a tumor microenvironment. Additionally, to investigate anti-tumor immune responses in vivo, particularly focusing on a tumor microenvironment, we developed mouse models and explored the pattern of the immunological phenotype according to the development/regression of tumors. Further, we are also developing novel Chimeric Antigen Receptor (CAR) T cells to improve the function and treatment outcome and are preparing for an investigator initiated clinical trial.
Clinical trials
We have collaborated with several pharmaceutical companies in their clinical trials to elucidate predictive biomarkers and immune status in a tumor microenvironment using our real-time immune monitoring system.
Education
Many graduate school students are trained in our division, and young residents in National Cancer Center East are also trained to become physician scientists. After finishing their thesis, they continue to study cancer immunology abroad.
Future prospects
Our division aims at elucidating the mechanisms involved in tumor immune suppression or tumor immune evasion in various types of cancers with a broad view such as immunology and genetics. In addition, investigation of immune responses has been performed in detail by integrated analyses using not only immunological methods, but also single-cell RNA seq and gene-editing. We pursue novel effective cancer immunotherapies and identify promising biomarkers based on the insights obtained from our fundamental research. We are also developing novel Chimeric Antigen Receptor (CAR) T cells to improve the treatment outcome and are preparing for an investigator initiated clinical trial.
List of papers published in 2019
Journal
1. Sasaki A, Nakamura Y, Togashi Y, Kuno H, Hojo H, Kageyama S, Nakamura N, Takashima K, Kadota T, Yoda Y, Mishima S, Sawada K, Kotani D, Kawazoe A, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Yano T, Kobayashi T, Akimoto T, Nishikawa H, Shitara K. Enhanced tumor response to radiotherapy after PD-1 blockade in metastatic gastric cancer. Gastric Cancer, 23:893-903, 2020
2. Sugiyama E, Togashi Y, Takeuchi Y, Shinya S, Tada Y, Kataoka K, Tane K, Sato E, Ishii G, Goto K, Shintani Y, Okumura M, Tsuboi M, Nishikawa H. Blockade of EGFR improves responsiveness to PD-1 blockade in EGFR-mutated non-small cell lung cancer. Sci Immunol, 5:pii: eaav3937. doi: 10.1126/sciimmunol.aav3937., 2020
3. Tanaka A, Nishikawa H, Noguchi S, Sugiyama D, Morikawa H, Takeuchi Y, Ha D, Shigeta N, Kitawaki T, Maeda Y, Saito T, Shinohara Y, Kameoka Y, Iwaisako K, Monma F, Ohishi K, Karbach J, Jäger E, Sawada K, Katayama N, Takahashi N, Sakaguchi S. Tyrosine kinase inhibitor imatinib augments tumor immunity by depleting effector regulatory T cells. J Exp Med, 217:pii: e20191009. doi: 10.1084/jem.20191009, 2020
4. Rodriguez-Garcia A, Watanabe K, Guedan S. Analysis of Antitumor Effects of CAR-T Cells in Mice with Solid Tumors. Methods Mol Biol, 2086:251-271, 2020
5. Tanaka A, Nishikawa H, Noguchi S, Sugiyama D, Morikawa H, Takeuchi Y, Ha D, Shigeta N, Kitawaki T, Maeda Y, Saito T, Shinohara Y, Kameoka Y, Iwaisako K, Monma F, Ohishi K, Karbach J, Jäger E, Sawada K, Katayama N, Takahashi N, Sakaguchi S. Tyrosine kinase inhibitor imatinib augments tumor immunity by depleting effector regulatory T cells. J Exp Med, 217:e20191009. doi: 10.1084/jem.20191009, 2020
6. Minoura K, Abe K, Maeda Y, Nishikawa H, Shimamura T. Model-based cell clustering and population tracking for time-series flow cytometry data. BMC Bioinformatics, 20:633, 2019
7. Tokunaga A, Sugiyama D, Maeda Y, Warner AB, Panageas KS, Ito S, Togashi Y, Sakai C, Wolchok JD, Nishikawa H. Selective inhibition of low-affinity memory CD8+ T cells by corticosteroids. J Exp Med, 216:2701-2713, 2019
8. Doi T, Muro K, Ishii H, Kato T, Tsushima T, Takenoyama M, Oizumi S, Gemmoto K, Suna H, Enokitani K, Kawakami T, Nishikawa H, Yamamoto N. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin Cancer Res, 25:6614-6622, 2019
9. Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci, 110:2080-2089, 2019
10. Datta M, Coussens LM, Nishikawa H, Hodi FS, Jain RK. Reprogramming the Tumor Microenvironment to Improve Immunotherapy: Emerging Strategies and Combination Therapies. Am Soc Clin Oncol Educ Book, 39:165-174, 2019
11. Tanegashima T, Togashi Y, Azuma K, Kawahara A, Ideguchi K, Sugiyama D, Kinoshita F, Akiba J, Kashiwagi E, Takeuchi A, Irie T, Tatsugami K, Hoshino T, Eto M, Nishikawa H. Immune Suppression by PD-L2 against Spontaneous and Treatment-Related Antitumor Immunity. Clin Cancer Res, 25:4808-4819, 2019
12. Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, Morikawa H, Kawazoe A, Kinoshita T, Shitara K, Sakaguchi S, Nishikawa H. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A, 116:9999-10008, 2019
13. Kochin V, Nishikawa H. <Editors' Choice> Meddling with meddlers: curbing regulatory T cells and augmenting antitumor immunity. Nagoya J Med Sci, 81:1-18, 2019
