Annual Report 2019
Department of Omics Network
Masaru Katoh
Introduction
The Department of Omics Network is involved in innovation based on the balance between its main world-class projects and cutting-edge new projects. The FGF (PMIDs: 23696246, 27245147 and 30367139), Hedgehog (PMIDs: 19860666 and 31036756), Notch (PMIDs: 17143535 and 31894255) and WNT (PMIDs: 17634527 and 29048660) signaling cascades and the Forkhead-box (FOX) family of transcription factors (PMIDs: 15492844 and 23022474) have been the main projects of the Department. Angiogenesis, cell adhesion, epigenetics and immune evasion in the tumor microenvironment have been new projects of the Department. The Department is currently concentrating its resources on the Knowledge-Base Project on the FGF, Hedgehog, Notch and WNT signaling cascades to maintain competitiveness and sustainability in the global scientific community undergoing dynamic changes amid the COVID-19 pandemic.
Masaru Katoh has worked in the fields of clinical medicine (1986 - 1990), molecular biology (1990 - 2002) and genome science (2003 - 2014), and has been working in the field of precision medicine since 2015. Global-level networking and high-quality manuscript publication are driving forces for the Department and Masaru Katoh to carry out digital transformation (DX) toward the Post-Corona Era.
FGFR-targeted therapy
FGFRs are receptor tyrosine kinases with versatile physiological roles, such as angiogenesis, phosphate homeostasis and tissue repair, whereas genetic alterations in or protein overexpression of FGFR1, FGFR2, FGFR3 and FGFR4 drive human carcinogenesis. Small-molecule compounds and antibody-based drugs have been developed as investigational drugs targeting FGFR alterations. FGFR inhibitors, erdafitinib and pemigatinib, have been approved for the treatment of subsets of cancer patients by the US FDA, because the benefits of FGFR inhibitors have been demonstrated in clinical trials in patients with cholangiocarcinoma harboring FGFR2 fusions and urothelial carcinoma with FGFR3 alterations. However, the response rates of FGFR inhibitors in patients with other types of cancers were relatively low, and responses in patients without detectable FGFR alterations were also observed. The selection of patients who are most likely to benefit from treatment using RNA in situ hybridization and the use of FGFR inhibitors in combination with chemotherapeutic agents or immune checkpoint inhibitors are expected to improve response rates and avoid acquired resistance.
Notch-targeted therapy
NOTCH1, NOTCH2, NOTCH3 and NOTCH4 are transmembrane receptors that transduce juxtacrine signals of DLL or JAG families of Notch ligands. Canonical Notch signaling activates the transcription of BMI1, CCND1, CD44, CDKN1A, HES1, HEY1, MYC, NOTCH3, REST and TCF7 (TCF-1) in a cellular context-dependent manner, whereas non-canonical Notch signaling activates NF-κB and RAC1 and inhibits ATM-dependent DNA-damage response. Notch signaling is inactivated in small-cell lung cancer (SCLC) and squamous cell carcinomas (SCCs), and loss-of-function NOTCH1 mutations are early events during esophageal tumorigenesis. In contrast, Notch signaling is aberrantly activated in breast cancer, diffuse large B-cell lymphoma (DLBCL), non-small-cell lung cancer (NSCLC) and T-cell acute lymphoblastic leukemia (T-ALL), and gain-of-function NOTCH1 mutations are late events during T-cell leukemogenesis and B-cell lymphomagenesis. The Notch signaling network exerts oncogenic and tumor-suppressive effects in a cancer stage- or (sub)type-dependent manner. Small-molecule γ-secretase inhibitors (AL101, MRK-560, nirogacestat and others) and antibody-based biologics targeting Notch ligands or receptors [ABT-165, AMG 119, rovalpituzumab tesirine (Rova-T) and others] have been developed as investigational drugs. Phase III clinical trials of Rova-T for SCLC patients and a phase III clinical trial of nirogacestat for patients with desmoid tumors are ongoing. Integration of human intelligence, cognitive computing and explainable artificial intelligence is mandatory to construct a Notch-related knowledge-base and optimize Notch-targeted therapy for cancer patients (Figure 1).
Figure 1.Human intelligence, cognitive computing and explainable artificial intelligence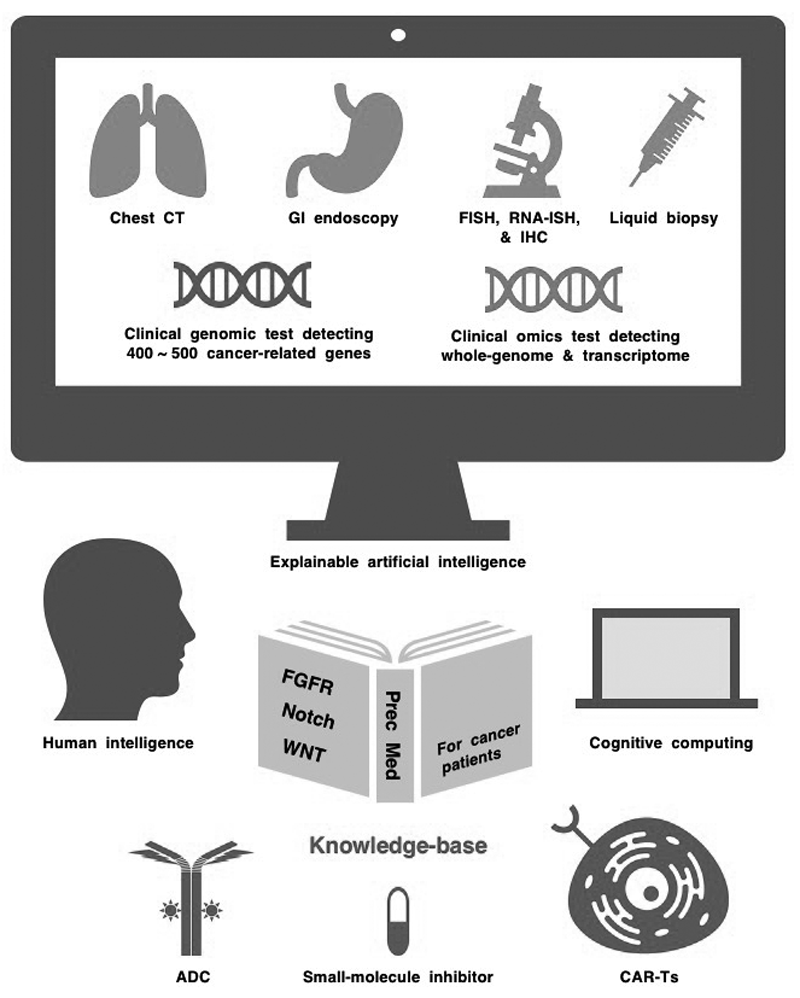

Global networking and contribution to the scientific community
Masaru Katoh has been contributing to the global scientific community through manuscript publication as well as reviewer and editor activities. Katoh is a member of the International Expert Panel of the National Medical Research Council (NMRC), a Singapore-based granting agency, and evaluated internationally competent grant proposals. He is an Academic Editor of an USA-based journal, PLoS ONE, and carried out editorial decisions 100 times in 2019. He is the Chief Editor of a Switzerland-based journal, Frontiers in Molecular Medicine, that aims to address the gap between cell and developmental biology and clinical medicine, together with 420 editorial board members.
The manuscript citation count in the Web of Science Database is a surrogate marker of the contribution to the global scientific community. Katoh’s manuscripts were cited approximately 600 times by others in 2019. Five manuscripts by Masaru Katoh are selected as “Highly Cited Papers” in the Web of Science Database. Katoh is the corresponding author of four of the “Highly Cited Papers” (PMIDs: 23022474, 23696246, 27245147 and 30367139).
List of papers published in 2019
Journal
1. Katoh M, Katoh M. Infigratinib. Drugs of the Future, 45:5-11, 2020
2. Katoh M, Katoh M. Precision medicine for human cancers with Notch signaling dysregulation (Review). Int J Mol Med, 45:279-297, 2020
3. Katoh M, Katoh M. CD157 and CD200 at the crossroads of endothelial remodeling and immune regulation. Stem Cell Investig, 6:10, 2019
4. Katoh M. Genomic testing, tumor microenvironment and targeted therapy of Hedgehog-related human cancers. Clin Sci (Lond), 133:953-970, 2019
