Annual Report 2020
Division of Translational Genomics (Tsukiji Campus)
Takashi Kohno, Hitoshi Ichikawa
Introduction
This division aims to facilitate cancer precision medicine by implementing next-generation sequencing (NGS)-based tumor profiling systems.
The Team and What We Do
This division organizes a clinical sequencing team with staff members from the Department of Experimental Therapeutics, Department of Diagnostic Pathology, Department of Laboratory Medicine and others in the National Cancer Center Hospitals. Our division aims to implement NGS-based tumor profiling systems, such as NCC Oncopanel and NCC Oncopanel-Ped.
Research activities
The NCC Oncopanel Test, which we developed as a basis for precision cancer medicine to select treatment methods based on genomic information, has been improved. The improved NCC Oncopanel test can detect mutations in 124 genes, microsatellite instability, tumor mutational burden (TMB), and germline mutations, and is being offered in more than 200 designated core and cooperative hospitals for cancer genomic medicine in Japan.
Based on the results of the NCC Oncopanel test, we found that maternal cervical cancer cells are transmitted through the amniotic fluid to the newborn's lungs at birth, causing pediatric lung cancer (Figure 1). We also found that cancer cells that are transmitted from the mother are highly responsive to immune checkpoint inhibition therapy (Figure 1). These results indicate that the introduction of cancer gene panel tests, such as the NCC Oncopanel, which we developed, into the medical field will enable the discovery of new cancer etiologies and the accurate understanding of its pathogenesis.
We have started to develop a borderless gene test that goes beyond the limitations of gene panel tests in terms of gene sets to be tested. This test will enable us to test any gene set using formalin-fixed paraffin-embedded cancer tissues, which are used in daily practice, as test materials.
Clinical trials
TOP-GEAR: Trial of Onco-Panel for Gene-profiling to Estimate both Adverse events and Response by Cancer Treatment (UMIN000011141)
Education
Post-doctoral fellows and chief residents in NCC have undergone on-the-job training in several translational research projects.
Figure 1. Immune checkpoint blockade therapy for cervical cancer transmitted from mother to infant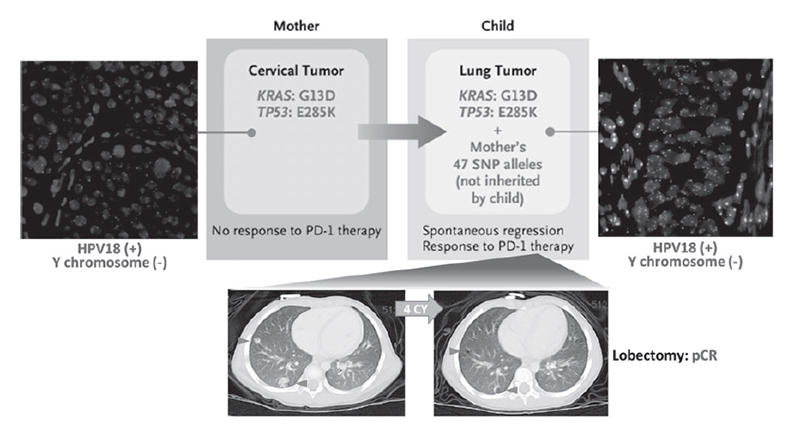

Future Prospects
Tumor-profiling gene panel tests underpin cancer genome medicine in Japan. However, there is still a gap between the number of patients with actionable mutations and those receiving genomically matched therapy. This gap is largely attributable to the lack of availability of/accessibility to relevant trials and drugs. To fill this gap, we are collaborating with oncologists for the following three purposes: 1) To facilitate molecularly driven clinical trials by implementing tumor genome profiling tests, 2) To annotate VUSs (variants of unknown significance mutations) in druggable genes and 3) To establish clinical trials for gene alterations with a status changing from “undruggable” to “druggable”, such as deleterious mutations in chromatin regulator genes.
List of papers published in 2020
Journal
1. Naito Y, Aburatani H, Amano T, Baba E, Furukawa T, Hayashida T, Hiyama E, Ikeda S, Kanai M, Kato M, Kinoshita I, Kiyota N, Kohno T, Kohsaka S, Komine K, Matsumura I, Miura Y, Nakamura Y, Natsume A, Nishio K, Oda K, Oda N, Okita N, Oseto K, Sunami K, Takahashi H, Takeda M, Tashiro S, Toyooka S, Ueno H, Yachida S, Yoshino T, Tsuchihara K. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (edition 2.1). Int J Clin Oncol, 26:233-283, 2021
2. Arakawa A, Ichikawa H, Kubo T, Motoi N, Kumamoto T, Nakajima M, Yonemori K, Noguchi E, Sunami K, Shiraishi K, Kakishima H, Yoshida H, Hishiki T, Kawakubo N, Kuroda T, Kiyokawa T, Yamada K, Yanaihara N, Takahashi K, Okamoto A, Hirabayashi S, Hasegawa D, Manabe A, Ono K, Matsuoka M, Arai Y, Togashi Y, Shibata T, Nishikawa H, Aoki K, Yamamoto N, Kohno T, Ogawa C. Vaginal Transmission of Cancer from Mothers with Cervical Cancer to Infants. N Engl J Med, 384:42-50, 2021
3. Watanabe S, Goto Y, Yasuda H, Kohno T, Motoi N, Ohe Y, Nishikawa H, Kobayashi SS, Kuwano K, Togashi Y. HSP90 inhibition overcomes EGFR amplification-induced resistance to third-generation EGFR-TKIs. Thorac Cancer, 12:631-642, 2021
4. Watanabe T, Honda T, Totsuka H, Yoshida M, Tanioka M, Shiraishi K, Shimada Y, Arai E, Ushiama M, Tamura K, Yoshida T, Kanai Y, Kohno T. Simple prediction model for homologous recombination deficiency in breast cancers in adolescents and young adults. Breast Cancer Res Treat, 182:491-502, 2020
5. Watanabe S, Shimomura A, Kubo T, Sekimizu M, Seo T, Watanabe SI, Kawai A, Yamamoto N, Tamura K, Kohno T, Ichikawa H, Yoshida A. BRAF V600E mutation is a potential therapeutic target for a small subset of synovial sarcoma. Mod Pathol, 33:1660-1668, 2020
