Annual Report 2020
Division of Cancer Immunology
Hiroyoshi Nishikawa, Shohei Koyama, Takuma Irie, Kota Itahashi, Yasuko Tada, Rika Fujii, Sho Watanabe, Tomomi Morioka, Shota Fukuoka, Koji Inaomori, Akihito Kawazoe, Daiki Sato, Nobuhiko Yamauchi, Junichiro Yuda, Shogo Kumagai, Tokiyoshi Tanegashima, Yi-tzu Lin, Atsuo Sai, Tamiyo Kobayashi, Sho Isoyama, Sayoko Shinya, Hidenori Kasahara, Konomi Onagawa, Tomoka Takaku, Megumi Takemura, Megumi Hoshino, Kumiko Yoshida, Mizuho Kikuchi, Yoko Ohira, Sayuri Yoshimatsu
Introduction
The Recent success of cancer immunotherapy, particularly immune checkpoint inhibitors (ICIs), makes it a key strategy for cancer treatment. While some patients who take ICIs experience long-lasting clinical benefits, more than half do not exhibit a clinical benefit. Moreover, certain patients experience recurrence after initially responding to such immunotherapy. There is therefore an urgent need to identify predictive biomarkers and develop optimal combination therapies with immunotherapies for individual patients.
The Team and What We Do
In our laboratory, we investigate cancer cells and immune cells in tumor microenvironments and peripheral blood using several techniques including multi-color flow-cytometry, multi-color immunohistochemistry, CyTOF, RNA sequencing, ATAC sequencing and whole exome sequencing in collaboration with the clinical department (Figure 1). Real-time immune monitoring in cancer patients while undergoing treatment has been established, through which we have collaborated with pharmaceutical companies in their clinical trials. In particular, regulatory T cells (Treg), which have a strong immune suppressive function and are a critical component for inhibiting anti-tumor immunity, are analyzed with our original method. With the findings from clinical specimens, we perform reverse translational research to elucidate the essential mechanisms of immune responses to cancer.
Figure 1. We are investigating the dynamic immune state in cancer patients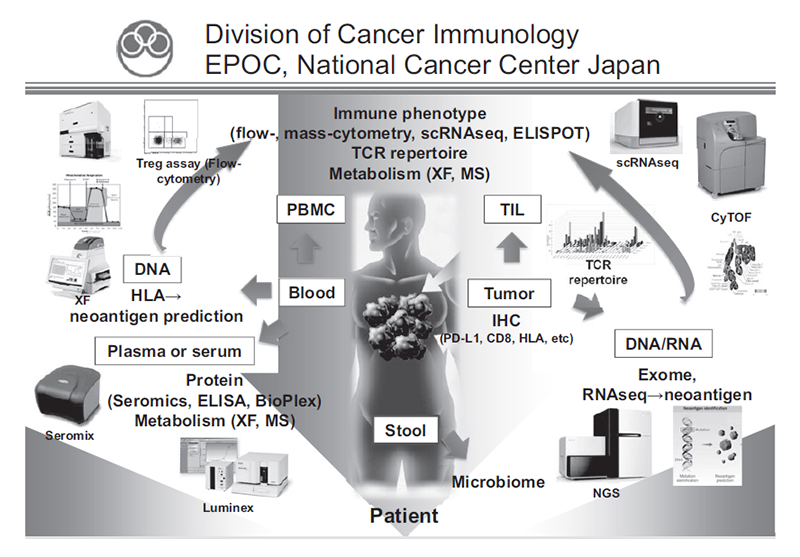

Research activities
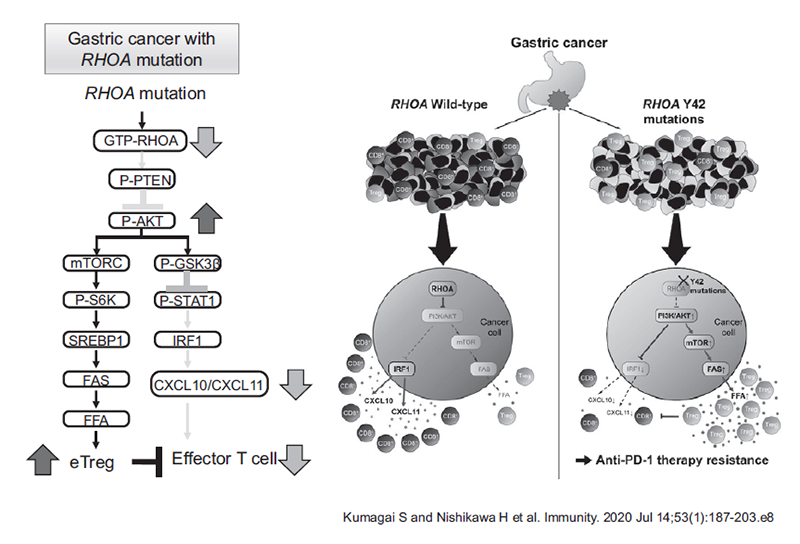
When the data from immune profiling in tumor microenvironments were integrated with genomic analyses in gastric cancer, we found that Rho-A mutation led to a Treg-rich immune microenvironment in gastric cancer through fatty acid production (Kumagai S et al. Immunity. 2020 Jul 14;53(1):187-203.e8) (Figure 2). Through our real-time immune monitoring system, we addressed kinetic changes in immune status in the tumor microenvironment after cancer therapies including ICIs and cytotoxic reagents. We discovered a reduction in activated Treg cells by a first-in-human clinical reagent using our real-time immune monitoring system, thereby proposing a novel effect of this drug.
Furthermore, the combination of two factors, PD-1 positivity in CD8 and effector Treg (eTreg), has been identified as a promising predictive biomarker in patients treated with immune checkpoint inhibitors. A subset of patients in Group R: PD-1+CD8+TILs>40% and PD-1 expression ratio CD8T/eTreg>1 showed much better outcomes for ICI treatment in the patients with lung cancer, gastric cancer and melanoma. (Kumagai S et al. Nat Immunol. 2020 Nov;21(11): 1346-1358.) (Figure 3).
Figure 3. Optimizing cancer immunotherapies based on biomarkers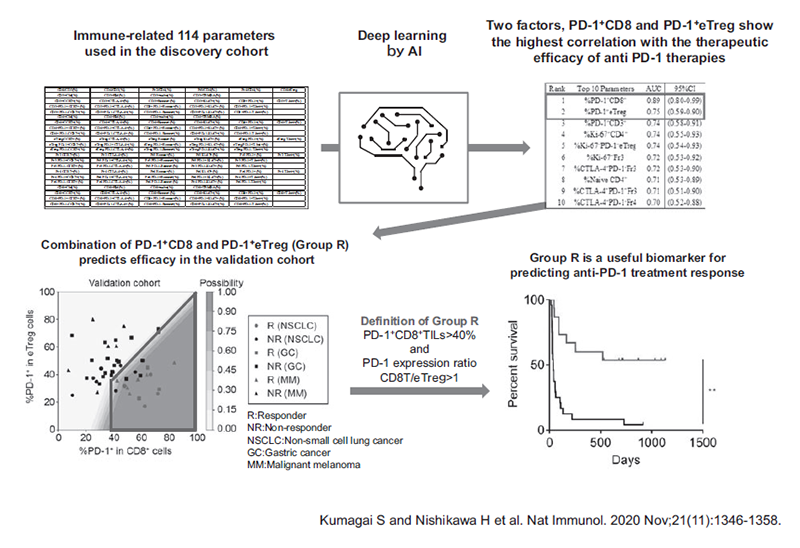

Clinical trials
We have collaborated with several pharmaceutical companies in their clinical trials to elucidate biomarkers for predicting the therapeutic response and to evaluate the immune status in tumor microenvironments using our real-time immune monitoring.
Education
Many graduate school students are trained in our division, and young residents in National Cancer Center East are also trained to become physician scientists. After finishing their PhD course, some of the students continue their cancer immunology studies abroad.
Future Prospects
ICIs have undoubtedly opened a new era in cancer treatment. However, there are still several issues, including acquired resistance and suppressive immune modulation by somatic mutations in cancer cells, for overcoming the drawbacks of ICIs. To answer these questions, immunological analyses using clinical specimens are essential. It is therefore important to develop better therapeutic strategies combining ICIs with other anti-cancer drugs and to identify biomarkers that can differentiate responders from non-responders by clarifying the immune suppressive networks in TMEs.
List of papers published in 2020
Journal
1. Arakawa A, Ichikawa H, Kubo T, Motoi N, Kumamoto T, Nakajima M, Yonemori K, Noguchi E, Sunami K, Shiraishi K, Kakishima H, Yoshida H, Hishiki T, Kawakubo N, Kuroda T, Kiyokawa T, Yamada K, Yanaihara N, Takahashi K, Okamoto A, Hirabayashi S, Hasegawa D, Manabe A, Ono K, Matsuoka M, Arai Y, Togashi Y, Shibata T, Nishikawa H, Aoki K, Yamamoto N, Kohno T, Ogawa C. Vaginal Transmission of Cancer from Mothers with Cervical Cancer to Infants. N Engl J Med, 384:42-50, 2021
2. Kumagai S, Koyama S, Nishikawa H. Antitumour immunity regulated by aberrant ERBB family signalling. Nat Rev Cancer, 21:181-197, 2021
3. Inamori K, Togashi Y, Fukuoka S, Akagi K, Ogasawara K, Irie T, Motooka D, Kobayashi Y, Sugiyama D, Kojima M, Shiiya N, Nakamura S, Maruyama S, Suzuki Y, Ito M, Nishikawa H. Importance of lymph node immune responses in MSI-H/dMMR colorectal cancer. JCI Insight, 6:2021
4. Seo W, Jerin C, Nishikawa H. Transcriptional regulatory network for the establishment of CD8+ T cell exhaustion. Exp Mol Med, 53:202-209, 2021
5. Kashima Y, Togashi Y, Fukuoka S, Kamada T, Irie T, Suzuki A, Nakamura Y, Shitara K, Minamide T, Yoshida T, Taoka N, Kawase T, Wada T, Inaki K, Chihara M, Ebisuno Y, Tsukamoto S, Fujii R, Ohashi A, Suzuki Y, Tsuchihara K, Nishikawa H, Doi T. Potentiality of multiple modalities for single-cell analyses to evaluate the tumor microenvironment in clinical specimens. Sci Rep, 11:341, 2021
6. Nozaki K, Fujioka Y, Sugiyama D, Ishikawa J, Iida M, Shibata M, Kosugi S, Nishikawa H, Shibayama H. Flow cytometry analysis of peripheral Tregs in patients with multiple myeloma under lenalidomide maintenance. Int J Hematol, 113:772-774, 2021
7. Chen S, Kiguchi T, Nagata Y, Tamai Y, Ikeda T, Kajiya R, Ono T, Sugiyama D, Nishikawa H, Akatsuka Y. A simple method to distinguish residual elotuzumab from monoclonal paraprotein in immunofixation assays for multiple myeloma patients. Int J Hematol, 113:473-479, 2021
8. Watanabe S, Goto Y, Yasuda H, Kohno T, Motoi N, Ohe Y, Nishikawa H, Kobayashi SS, Kuwano K, Togashi Y. HSP90 inhibition overcomes EGFR amplification-induced resistance to third-generation EGFR-TKIs. Thorac Cancer, 12:631-642, 2021
9. Muramatsu T, Noguchi T, Sugiyama D, Kanada Y, Fujimaki K, Ito S, Gotoh M, Nishikawa H. Newly emerged immunogenic neoantigens in established tumors enable hosts to regain immunosurveillance in a T-cell-dependent manner. Int Immunol, 33:39-48, 2021
10. Bando H, Kotani D, Tsushima T, Hara H, Kadowaki S, Kato K, Chin K, Yamaguchi K, Kageyama SI, Hojo H, Nakamura M, Tachibana H, Wakabayashi M, Fukutani M, Togashi Y, Fuse N, Nishikawa H, Kojima T. TENERGY: multicenter phase II study of Atezolizumab monotherapy following definitive Chemoradiotherapy with 5-FU plus Cisplatin in patients with unresectable locally advanced esophageal squamous cell carcinoma. BMC Cancer, 20:336, 2020
11. Kawazoe A, Kuboki Y, Shinozaki E, Hara H, Nishina T, Komatsu Y, Yuki S, Wakabayashi M, Nomura S, Sato A, Kuwata T, Kawazu M, Mano H, Togashi Y, Nishikawa H, Yoshino T. Multicenter Phase I/II Trial of Napabucasin and Pembrolizumab in Patients with Metastatic Colorectal Cancer (EPOC1503/SCOOP Trial). Clin Cancer Res, 26:5887-5894, 2020
12. Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, Sato E, Kuwata T, Kinoshita T, Yamamoto M, Nomura S, Tsukamoto T, Mano H, Shitara K, Nishikawa H. An Oncogenic Alteration Creates a Microenvironment that Promotes Tumor Progression by Conferring a Metabolic Advantage to Regulatory T Cells. Immunity, 53:187-203.e8, 2020
13. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y, Matsui S, Shibahara T, Yamashita Y, Irie T, Tsuge A, Fukuoka S, Kawazoe A, Udagawa H, Kirita K, Aokage K, Ishii G, Kuwata T, Nakama K, Kawazu M, Ueno T, Yamazaki N, Goto K, Tsuboi M, Mano H, Doi T, Shitara K, Nishikawa H. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol, 21:1346-1358, 2020
14. Umemoto K, Togashi Y, Arai Y, Nakamura H, Takahashi S, Tanegashima T, Kato M, Nishikawa T, Sugiyama D, Kojima M, Gotohda N, Kuwata T, Ikeda M, Shibata T, Nishikawa H. The potential application of PD-1 blockade therapy for early-stage biliary tract cancer. Int Immunol, 32:273-281, 2020
15. Nagasaki J, Togashi Y, Sugawara T, Itami M, Yamauchi N, Yuda J, Sugano M, Ohara Y, Minami Y, Nakamae H, Hino M, Takeuchi M, Nishikawa H. The critical role of CD4+ T cells in PD-1 blockade against MHC-II-expressing tumors such as classic Hodgkin lymphoma. Blood Adv, 4:4069-4082, 2020
16. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol, 38:2053-2061, 2020
17. Minoura K, Abe K, Maeda Y, Nishikawa H, Shimamura T. CYBERTRACK2.0: zero-inflated model-based cell clustering and population tracking method for longitudinal mass cytometry data. Bioinformatics, 2020
18. Abe K, Minoura K, Maeda Y, Nishikawa H, Shimamura T. Model-based clustering for flow and mass cytometry data with clinical information. BMC Bioinformatics, 21:393, 2020
19. Murate K, Maeda K, Nakamura M, Sugiyama D, Wada H, Yamamura T, Sawada T, Mizutani Y, Ishikawa T, Furukawa K, Ohno E, Honda T, Kawashima H, Miyahara R, Ishigami M, Nishikawa H, Fujishiro M. Endoscopic Activity and Serum TNF-α Level at Baseline Are Associated With Clinical Response to Ustekinumab in Crohn's Disease Patients. Inflamm Bowel Dis, 26:1669-1681, 2020
