Annual Report 2020
Section of Data Science Strategy
Takashi Kohno, Hirokazu Fukuda, Katsuya Tsuchihara, Genta Ohno, Haruka Nakada
The Team and What We Do
The mission of this section is to establish policies on how to collect and utilize genomic and clinical data of patients who underwent gene panel tests under the National Health Insurance System. The first one is on informed consent (IC) and it is established through a joint effort with Designated Core Hospitals for Cancer Genome Medicine. The model IC form and S.O.P. for gene panel tests were prepared through discussion. The second one is to provide the collected genomic and clinical data for researchers of industrial and academic institutions in Japan and abroad. This section will conduct a review board to provide the C-CAT data to those parties.
Activities
The services of portal site system to share C-CAT data with faculties of the Core and Liaison Hospitals for Cancer Genome Medicine have been started (Figure 1). The basic design of a portal site system to share C-CAT data with researchers of industrial and academic institutions has also been made. “The C-CAT Data Utilization Review Board” has been established, prior to the launch of the C-CAT data utilization service scheduled in 2021. A new website was opened, that provides information on caner genomic medicine and tumor profiling test.
Figure 1: Medical and Research Use of C-CAT “Real World” Data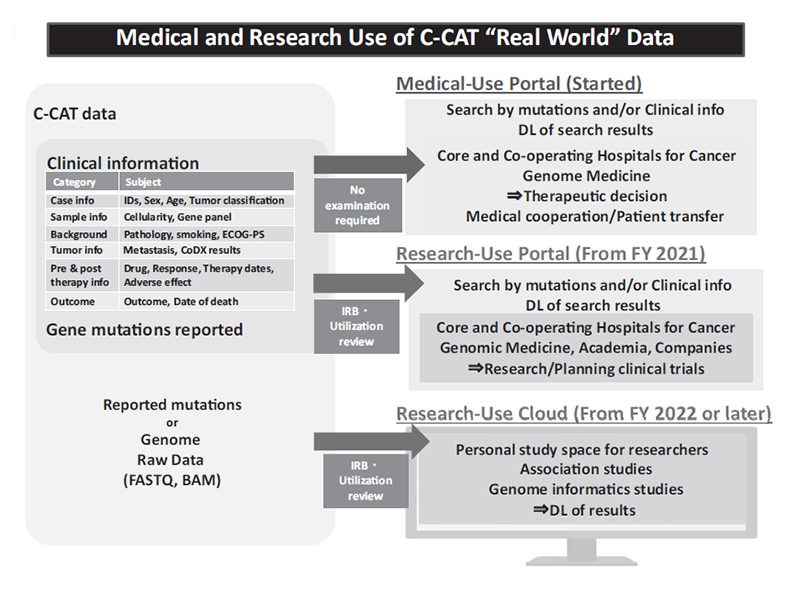

Future Prospects
Construction of “C-CAT data utilization portal site” to share C-CAT data with researchers of industrial and academic institutions will be completed, and the C-CAT data utilization service will be started in 2021. The basic design of a secure platform for researchers of industrial and academic institutions to analyze the relationship between medical and genomic data of C-CAT will be made.
