Annual Report 2020
Department of Hepatobiliary and Pancreatic Oncology
Masafumi Ikeda, Shuichi Mitsunaga, Hiroshi Imaoka, Yusuke Hashimoto, Mitsuhito Sasaki, Kazuo Watanabe, Shoichi Miyazawa, Toru Otsuru, Taro Shibuki, Kohei Hayashi, Hiroki Eguchi, Kyosuke Goda
Introduction
The Department of Hepatobiliary and Pancreatic Oncology is responsible for the diagnosis and treatment of patients with hepatic, biliary, and pancreatic cancers as well as interventional management by endoscopic or percutaneous procedures (Table 1). Our goal is to provide high-quality cancer treatment with adequate palliative care, and to develop novel and effective treatments and procedures through well-designed clinical trials and research.
Table 1. Number of cancer patients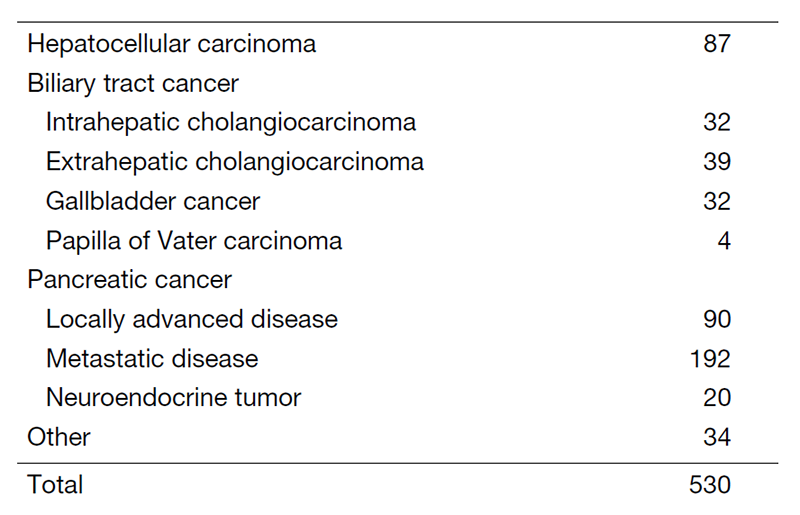

The Team and What We Do
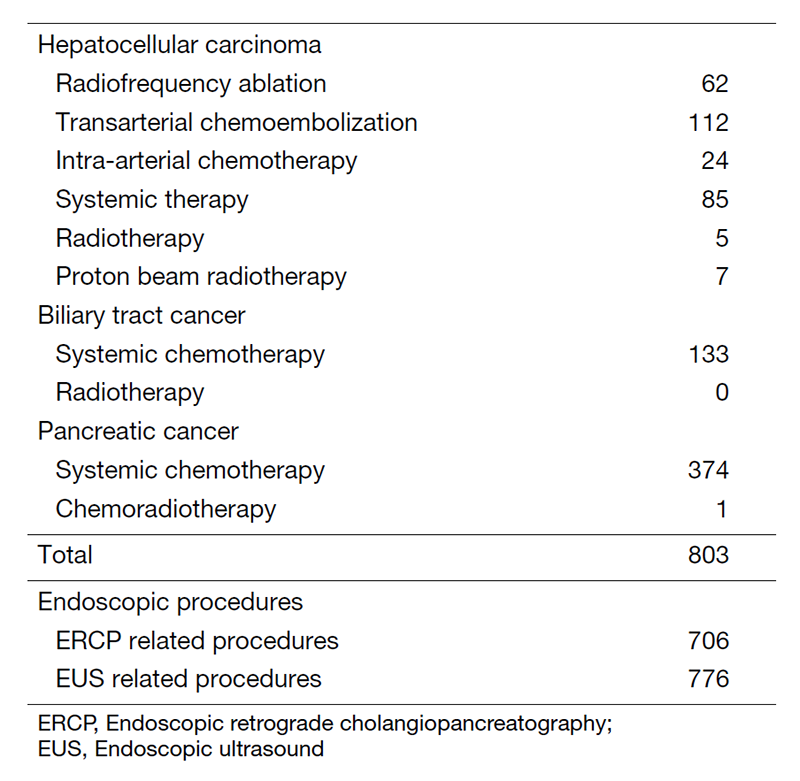
Our department is composed of 7 staff oncologists and 5 residents, with an average of 45 beds in the hospital. These doctors are divided into two teams and each team determines daily treatment plans for each admitted patient. The major treatment strategies for individual patients are discussed in weekly tumor board conferences attended by medical oncologists, surgeons, radiologists, radiation oncologists, and pharmacists. We are also responsible for endoscopic abdominal ultrasonographic examinations, endoscopic or percutaneous ultrasound-guided biopsies of abdominal masses, local ablative therapy for liver tumors, endoscopic or percutaneous biliary drainage, abscess drainage and stenting for obstructive jaundice, etc (Table 2).
Research activities
Hepatocellular carcinoma (HCC)
Systemic therapy for hepatobiliary and pancreatic cancer has changed dramatically in recent years. In patients with advanced hepatocellular carcinoma, cancer immunotherapy of atezolizumab plus bevacizumab has been reported to be superior to sorafenib in terms of overall survival and progression-free survival. This therapy replaced the standard first-line treatment, and immune checkpoint inhibitors have been first introduced in the field of HCC. Furthermore, cabozantinib was reimbursed in patients with HCC after second-line treatment. In the future, how to appropriately use the agents, including sorafenib, lenvatinib, regorafenib, ramucirumab and cabozantinib, will also be one of the important clinical issues.
Biliary tract cancer (BTC)
In biliary tract cancer, the development of molecular targeted agents for genome alterations has been successful, and the following agents have shown favorable efficacy in clinical trials: futibatinib and pemigatinib for FGFR fusion, ivosidenib for IDH1 mutation, and dabrafenib plus trametinib for BRAF V600E mutation. Furthermore, an HER2 inhibitor for HER2 amplification is under development. The development of immune checkpoint inhibitors is also underway. In the future, it is expected that precision oncology will become mainstream for BTC.
Pancreatic cancer (PC)
For pancreatic cancer, nanoliposomal irinotecan, which is a drug delivery system formation, has been approved in Japan and is being actively used after the second-line treatment of pancreatic cancer. In addition, olaparib as maintenance chemotherapy after treatment with platinum-based regimen is approved for patients with unresectable pancreatic cancer with germline BRCA mutation, and oncologists treating patients with pancreatic cancer, as well as oncologists treating patients with breast cancer and ovarian cancer, face a situation that requires genetic counseling. To date, the development of cytotoxic anticancer agents has been the primary target, but new targets are also being developed in hepatobiliary and pancreatic cancers, including immune checkpoint inhibitors and targeted therapies for genomic alterations, including germline mutations.
Clinical trials
A total of 90 clinical trials (sponsored: 45 trials, investigator-initiated: 45 trials) are ongoing, and 13 clinical trials (sponsored: eight trials, investigator-initiated: five trials) are being planned for the upcoming year.
HCC
Rapid advances have been made in systemic therapy for advanced HCC, and currently, six regimens, including first-line treatment with sorafenib, lenvatinib, and atezolizumab plus bevacizumab and second-line treatment, after sorafenib failure, with regorafenib, ramucirumab (only patients with serum α-fetoprotein levels of ≥400 ng/ml), and cabozantinib are reimbursable for the treatment of HCC in Japan. In clinical practice, various patterns of sequential treatment are considered, and how to appropriately use these agents is also one of the important clinical issues. Therefore, we planned to conduct a prospective observational study to resolve various clinical questions related to systemic therapy for advanced HCC in Japan (PRISM study; planed sample size 1000 patients, enrollment 2 years).
The enrollment of investigator-initiated phase II trial of lenvatinib plus intra-arterial cisplatin and some sponsored trials of pembrolizumab plus lenvatinib was closed. A global phase III trial of ipilimumab plus nivolumab versus sorafenib, phase I trial of triplet regimen of atezolizumab plus bevacizumb plus oncolytic virus (OPB301), or an anti-glypican3/CD3 bispecific antibody (ERY974) as the first-line therapy, and a phase I trial of lenvatinib plus beta-catenin modulator (E7386), and as the second- or later-line therapy, are underway.
BTC
The enrollment of some sponsored trials of GC plus immune checkpoint inhibitors, including durvalumab, bintrafusp alfa and pembrolizumab, as first-line chemotherapy and some sponsored trials of nivolumab plus lenvatinib, DS8201a for HER2-positive, etc., for advanced BTCs refractory to gemcitabine (Gem)-based chemotherapy was terminated. Some sponsored trials of JPH203 for anti-cancer drug targeting L-type amino acid transporter 1, E7090 for FGFR2 fusion, and LY3410738 for IDH1 mutation, etc., for second- or later-line advanced BTCs were conducted. For patients with occupational cholangiocarcinoma, a phase II trial of nivolumab is also ongoing as an investigator-initiated trial.
PC
Some clinical trials of a phase II/III trial of neoadjuvant S-1 and concurrent radiotherapy versus Gem plus nab-paclitaxel for borderline resectable PC (GABARNANCE) and Gem plus nab-paclitaxel versus modified FOLFIRINOX versus S-IROX for metastatic PC (JCOG1611), as the first-line setting, are ongoing. Some sponsored trials of FOLFIRINOX or Gem plus nab-paclitaxel with molecular targeted agents or immune checkpoint inhibitor are being planned.
Neuroendocrine tumor
A phase III trial of everolimus plus lanreotide versus everolimus is ongoing for gastroenterol and pancreatic neuroendocrine tumor.
Others
Some investigator-initiated studies using circulating tumor DNA are ongoing for hepatobiliary and pancreatic cancer.
Education
For our residents, daily training is provided with group discussions on the daily practice of management of inpatients and outpatients. They can learn the indications, administration and the management of adverse events from loco-regional treatments to systemic chemotherapy for patients with hepatic, biliary, and pancreatic cancer and the accompanying procedures to undertake diagnosis and interventional management, and so on. In addition, they can make presentations of their research in domestic and overseas meetings and publish papers in English under the instruction of staff physicians.
Future Prospects
The prognosis of patients with hepatic, biliary, and pancreatic cancers remains dismal, and the efficacy of standard treatments for these cancer is limited. In Japan, the incidences of these cancers, especially HCC and BTC, are higher than those in Western countries. Therefore, it is necessary to conduct a lot of top-level novel and promising clinical trials and research. It is also vital to develop biomarker research and endoscopic management alongside cancer treatment.
List of papers published in 2020
Journal
1. Maruki Y, Morizane C, Arai Y, Ikeda M, Ueno M, Ioka T, Naganuma A, Furukawa M, Mizuno N, Uwagawa T, Takahara N, Kanai M, Asagi A, Shimizu S, Miyamoto A, Yukisawa S, Kadokura M, Kojima Y, Furuse J, Nakajima TE, Sudo K, Kobayashi N, Hama N, Yamanaka T, Shibata T, Okusaka T. Molecular detection and clinicopathological characteristics of advanced/recurrent biliary tract carcinomas harboring the FGFR2 rearrangements: a prospective observational study (PRELUDE Study). J Gastroenterol, 56:250-260, 2021
2. Ioka T, Furuse J, Fukutomi A, Mizusawa J, Nakamura S, Hiraoka N, Ito Y, Katayama H, Ueno M, Ikeda M, Sugimori K, Okano N, Shimizu K, Yanagimoto H, Okusaka T, Ozaka M, Todaka A, Nakamori S, Tobimatsu K, Sata N, Kawashima Y, Hosokawa A, Yamaguchi T, Miyakawa H, Hara H, Mizuno N, Ishii H. Randomized phase II study of chemoradiotherapy with versus without induction chemotherapy for locally advanced pancreatic cancer: Japan Clinical Oncology Group trial, JCOG1106. Jpn J Clin Oncol, 51:235-243, 2021
3. Ikeda M, Okusaka T, Ohno I, Mitsunaga S, Kondo S, Ueno H, Morizane C, Gemmoto K, Suna H, Ushida Y, Furuse J. Phase I studies of peptide vaccine cocktails derived from GPC3, WDRPUH and NEIL3 for advanced hepatocellular carcinoma. Immunotherapy, 13:371-385, 2021
4. Takahashi H, Ikeda M, Shiba S, Imaoka H, Todaka A, Shioji K, Yane K, Kojima Y, Kobayashi S, Asagi A, Ozaka M, Takada R, Nagashio Y, Horiguchi S, Kasuga A, Suzuki E, Terashima T, Ueno M, Morizane C, Furuse J. Multicenter Retrospective Analysis of Chemotherapy for Advanced Pancreatic Acinar Cell Carcinoma: Potential Efficacy of Platinum- and Irinotecan-Containing Regimens. Pancreas, 50:77-82, 2021
5. Umemoto K, Takahashi H, Morizane C, Yamada I, Shimizu S, Shioji K, Yoshida Y, Motoya M, Mizuno N, Kojima Y, Terashima T, Uesugi K, Ueno M, Furuse J, Akimoto T, Ikeda M. FOLFIRINOX in advanced pancreatic cancer patients with the double-variant type of UGT1A1 *28 and *6 polymorphism: a multicenter, retrospective study. Cancer Chemother Pharmacol, 87:397-404, 2021
6. Kudo M, Tsuchiya K, Kato N, Hagihara A, Numata K, Aikata H, Inaba Y, Kondo S, Motomura K, Furuse J, Ikeda M, Morimoto M, Achira M, Kuroda S, Kimura A. Cabozantinib in Japanese patients with advanced hepatocellular carcinoma: a phase 2 multicenter study. J Gastroenterol, 56:181-190, 2021
7. Nakamura Y, Taniguchi H, Ikeda M, Bando H, Kato K, Morizane C, Esaki T, Komatsu Y, Kawamoto Y, Takahashi N, Ueno M, Kagawa Y, Nishina T, Kato T, Yamamoto Y, Furuse J, Denda T, Kawakami H, Oki E, Nakajima T, Nishida N, Yamaguchi K, Yasui H, Goto M, Matsuhashi N, Ohtsubo K, Yamazaki K, Tsuji A, Okamoto W, Tsuchihara K, Yamanaka T, Miki I, Sakamoto Y, Ichiki H, Hata M, Yamashita R, Ohtsu A, Odegaard JI, Yoshino T. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med, 26:1859-1864, 2020
8. Ikeda M, Morizane C, Hijioka S, Matsumoto S, Konishi T, Komoto I, Aoki T, Ito T, Furuse J, Sasano H, Doi R. Optimal strategy of systemic treatment for unresectable pancreatic neuroendocrine tumors based upon opinion of Japanese experts. Pancreatology, 20:944-950, 2020
9. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut, 69:1492-1501, 2020
10. Kudo M, Morimoto M, Moriguchi M, Izumi N, Takayama T, Yoshiji H, Hino K, Oikawa T, Chiba T, Motomura K, Kato J, Yasuchika K, Ido A, Sato T, Nakashima D, Ueshima K, Ikeda M, Okusaka T, Tamura K, Furuse J. A randomized, double-blind, placebo-controlled, phase 3 study of tivantinib in Japanese patients with MET-high hepatocellular carcinoma. Cancer Sci, 111:3759-3769, 2020
11. Okano N, Morizane C, Nomura S, Takahashi H, Tsumura H, Satake H, Mizuno N, Tsuji K, Shioji K, Asagi A, Yasui K, Kitagawa S, Kashiwada T, Ishiguro A, Kanai M, Ueno M, Ogura T, Shimizu S, Tobimatsu K, Motoya M, Nakashima K, Ikeda M, Okusaka T, Furuse J. Phase II clinical trial of gemcitabine plus oxaliplatin in patients with metastatic pancreatic adenocarcinoma with a family history of pancreatic/breast/ovarian/prostate cancer or personal history of breast/ovarian/prostate cancer (FABRIC study). Int J Clin Oncol, 25:1835-1843, 2020
12. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol, 38:2960-2970, 2020
13. Tanaka H, Hijioka S, Hosoda W, Ueno M, Kobayashi N, Ikeda M, Ito T, Kodama Y, Morizane C, Notohara K, Taguchi H, Kitano M, Komoto I, Tsuji A, Hashigo S, Kanno A, Miyabe K, Takagi T, Ishii H, Kojima Y, Yoshitomi H, Yanagimoto H, Furuse J, Mizuno N. Pancreatic neuroendocrine carcinoma G3 may be heterogeneous and could be classified into two distinct groups. Pancreatology, 20:1421-1427, 2020
14. Ueno M, Nakamori S, Sugimori K, Kanai M, Ikeda M, Ozaka M, Furukawa M, Okusaka T, Kawabe K, Furuse J, Komatsu Y, Ishii H, Sato A, Shimizu S, Chugh P, Tang R, Ioka T. nal-IRI+5-FU/LV versus 5-FU/LV in post-gemcitabine metastatic pancreatic cancer: Randomized phase 2 trial in Japanese patients. Cancer Med, 9:9396-9408, 2020
15. Imaoka H, Ikeda M, Maehara K, Umemoto K, Ozaka M, Kobayashi S, Terashima T, Inoue H, Sakaguchi C, Tsuji K, Shioji K, Okamura K, Kawamoto Y, Suzuki R, Shirakawa H, Nagano H, Ueno M, Morizane C, Furuse J. Clinical outcomes of chemotherapy in patients with undifferentiated carcinoma of the pancreas: a retrospective multicenter cohort study. BMC Cancer, 20:946, 2020
16. Umemoto K, Togashi Y, Arai Y, Nakamura H, Takahashi S, Tanegashima T, Kato M, Nishikawa T, Sugiyama D, Kojima M, Gotohda N, Kuwata T, Ikeda M, Shibata T, Nishikawa H. The potential application of PD-1 blockade therapy for early-stage biliary tract cancer. Int Immunol, 32:273-281, 2020
17. Sugimoto M, Kobayashi T, Kobayashi S, Takahashi S, Konishi M, Mitsunaga S, Ikeda M, Gotohda N. Extrapancreatic Nerve Plexus Invasion on Imaging Predicts Poor Survival After Upfront Surgery for Anatomically Resectable Pancreatic Cancer. Pancreas, 49:675-682, 2020
18. Takahashi D, Kojima M, Morisue R, Sugimoto M, Kobayashi S, Takahashi S, Konishi M, Gotohda N, Ikeda M, Ochiai A. Comparison of morphological features in lymph node metastasis between pancreatic neuroendocrine neoplasms and pancreatic ductal adenocarcinomas. Pancreatology, 20:936-943, 2020
19. Okuyama H, Ikeda M, Okusaka T, Furukawa M, Ohkawa S, Hosokawa A, Kojima Y, Hara H, Murohisa G, Shioji K, Asagi A, Mizuno N, Kojima M, Yamanaka T, Furuse J. A Phase II Trial of Everolimus in Patients with Advanced Pancreatic Neuroendocrine Carcinoma Refractory or Intolerant to Platinum-Containing Chemotherapy (NECTOR Trial). Neuroendocrinology, 110:988-993, 2020
20. Watanabe K, Mitsunaga S, Kojima M, Suzuki H, Irisawa A, Takahashi H, Sasaki M, Hashimoto Y, Imaoka H, Ohno I, Ikeda M, Akimoto T, Ochiai A. The "histological replacement growth pattern" represents aggressive invasive behavior in liver metastasis from pancreatic cancer. Cancer Med, 9:3130-3141, 2020
21. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med, 382:1894-1905, 2020
22. Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, Wang CK, Ikeda M, Chan SL, Choo SP, Miyayama S, Cheng AL. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer, 9:245-260, 2020
23. Kudo M, Kurosaki M, Ikeda M, Aikata H, Hiraoka A, Torimura T, Sakamoto N. Treatment of hepatocellular carcinoma during the COVID-19 outbreak: The Working Group report of JAMTT-HCC. Hepatol Res, 50:1004-1014, 2020
24. Kan M, Imaoka H, Watanabe K, Sasaki M, Takahashi H, Hashimoto Y, Ohno I, Mitsunaga S, Umemoto K, Kimura G, Suzuki Y, Eguchi H, Otsuru T, Goda K, Ikeda M. Chemotherapy-induced neutropenia as a prognostic factor in patients with pancreatic cancer treated with gemcitabine plus nab-paclitaxel: a retrospective cohort study. Cancer Chemother Pharmacol, 86:203-210, 2020
25. Ueshima K, Ogasawara S, Ikeda M, Yasui Y, Terashima T, Yamashita T, Obi S, Sato S, Aikata H, Ohmura T, Kuroda H, Ohki T, Nagashima K, Ooka Y, Takita M, Kurosaki M, Chayama K, Kaneko S, Izumi N, Kato N, Kudo M, Omata M. Hepatic Arterial Infusion Chemotherapy versus Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer, 9:583-595, 2020
26. Mitsunaga S, Kasamatsu E, Machii K. Incidence and frequency of cancer cachexia during chemotherapy for advanced pancreatic ductal adenocarcinoma. Support Care Cancer, 28:5271-5279, 2020
