Annual Report 2020
Department of Genetic Medicine and Services
Takeshi Kuwata, Kazuya Tsuchihara, Toru Mukohara, Akiko Nakayama, Sachiyo Mimaki, Kiwamu Akagi, Yumie Hiraoka, Kenichi Harano, Shingo Matsumoto, Nobuyuki Nakamura, Kyoko Tsuyoshi
Introduction
The Department of Genetic Medicine and Services was established in May 2016 for dealing with genetic as well as genomic testings and their related issues, including genetic counseling conducted in the National Cancer Center Hospital East (NCCHE).
The Team and What We Do
The aim of the Department of Genetic Medicine and Services is to deal with the following subjects:
1) Genetic counseling
2) Genetic/genomic testings
3) Ethical issues related to genetic/genomic medicine
4) Education of genetic/genomic medicine
5) Regulation of storage and usage of genetic/genomic information
6) Other genetic/genomic medicine
For this purpose, the members of the department were assembled with various specialties, including medical doctors, nurses, genetic counselor and medical technicians from different clinical departments or research laboratories.
The Outpatient Genetic Counseling Clinic provides genetic counseling and genetic testing for cancer patients and their relatives with familial history and/or specific features suspected for familial cancers. From April 2020 to March 2021, 105 new clients visited the clinic, and a total of 193 genetic counseling sessions were held and 65 genetic testings were provided. As a result, by March 2021, a total of 384 clients visited the clinic (Figures 1 and 2). The department also participated in outpatient clinic specialized for Hereditary Breast and Ovary Cancer (HBOC) syndrome established by the Department of Breast and Medical Oncology.
Figure 1. Number of genetic counseling sessions provided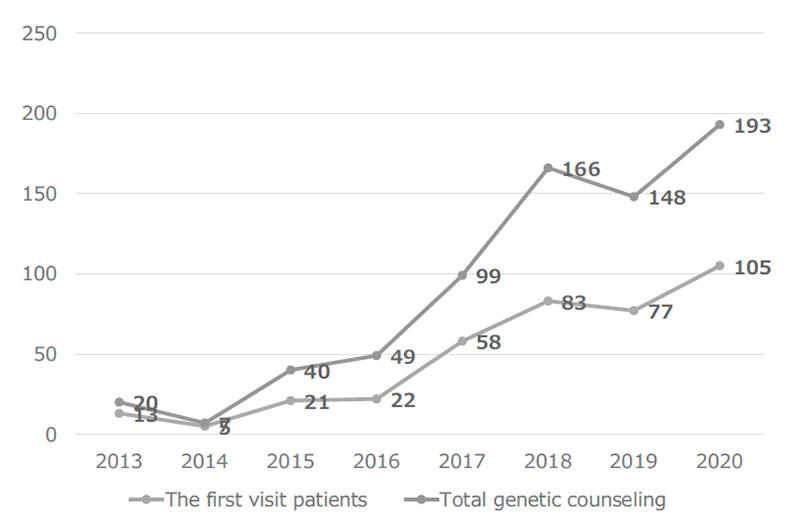

Figure 2. Breakdown of genetic counseling sessions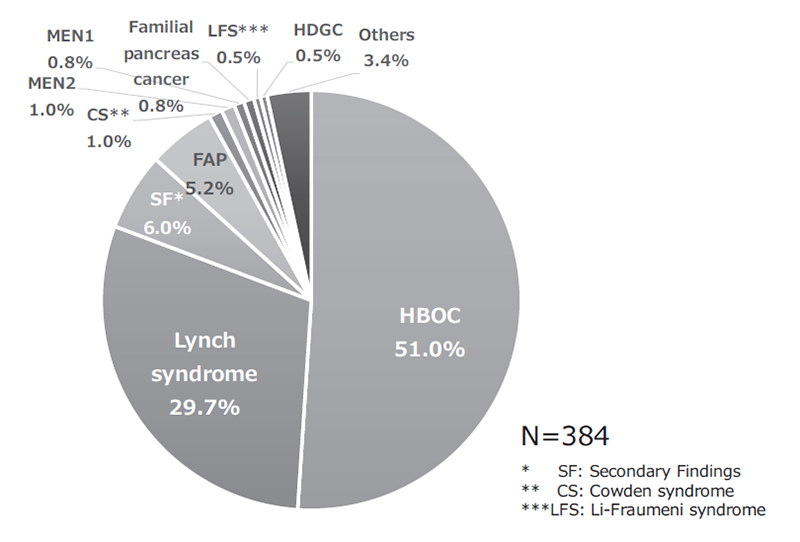

For conducting precision oncology, our hospital is designated as Cancer Genome Medical Core Hospitals in Japan and cooperated with 9 affiliated hospitals, especially for providing cancer genome profiling (CGP) tests. Under national health care services, by March 2021, we have performed 233 CGP tests and conducted expert panels for 236 patients, including 194 patients in affiliated hospitals.
Research activities
Our Outpatient Genetic Counseling Clinic is participating in the NCC Research and Development Fund program (25-A-1) and provides genetic testings.
Clinical trials
Our Outpatient Genetic Counseling Clinic supports and provides genetic counseling for patients willing to participate in clinical trials where genetic tests are required.
Education
We educated medical doctors who wish to become a board-certified geneticist by attending the Outpatient Genetic Counseling Clinic. We have also provided intramural educational seminars for medical doctors and paramedical personnel to make them familiar with genetic and genomic medicine to provide precision medicine for all the cancer patients and their families visiting our hospital. As Cancer Genome Medical Core Hospital, we also provide seminars and lectures for educating doctors as well as paramedical personnel working at affiliated hospitals.
Future Prospects
We will continue to provide genetic counseling and genetic testing for possible familial cancer patients/families. For precision oncology, we will conduct CGP tests under the national health care system and participate in several clinical trials using new technologies including liquid biopsy and whole genome/exome sequences. We will also continue to provide education programs for medical doctors and paramedics working in our hospital as well as affiliated hospitals. Our current aim is to establish a genomic testing pipeline from research to clinic for accelerating the development of medical and diagnostic devices for genome medicine.
List of papers published in 2020
Journal
1. Fujitani K, Nakamura K, Mizusawa J, Kuwata T, Shimoda T, Katayama H, Kushima R, Taniguchi H, Yoshikawa T, Boku N, Terashima M, Fukuda H, Sano T, Sasako M. Posttherapy topographical nodal status, ypN-site, predicts survival of patients who received neoadjuvant chemotherapy followed by curative surgical resection for non-type 4 locally advanced gastric cancer: supplementary analysis of JCOG1004-A. Gastric Cancer, 24:197-204, 2021
2. Yagishita S, Kato K, Takahashi M, Imai T, Yatabe Y, Kuwata T, Suzuki M, Ochiai A, Ohtsu A, Shimada K, Nishida T, Hamada A, Mano H. Characterization of the large-scale Japanese patient-derived xenograft (J-PDX) library. Cancer Sci, 112:2454-2466, 2021
3. Hasegawa Y, Matsubara N, Kogawa T, Naito Y, Harano K, Hosono A, Onishi T, Hojo T, Shimokawa M, Mukohara T. Neo-Bioscore in Guiding Post-surgical Therapy in Patients With Triple-negative Breast Cancer Who Received Neoadjuvant Chemotherapy. In Vivo, 35:1041-1049, 2021
4. Okamoto A, Kondo E, Nakamura T, Yanagida S, Hamanishi J, Harano K, Hasegawa K, Hirasawa T, Hori K, Komiyama S, Matsuura M, Nakai H, Nakamura H, Sakata J, Tabata T, Takehara K, Takekuma M, Yokoyama Y, Kase Y, Sumino S, Soeda J, Suri A, Aoki D, Sugiyama T. Phase 2 single-arm study on the efficacy and safety of niraparib in Japanese patients with heavily pretreated, homologous recombination-deficient ovarian cancer. J Gynecol Oncol, 32:e16, 2021
5. Amemiya R, Miyoshi T, Aokage K, Suzuki J, Hoshino H, Udagawa H, Tane K, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Goto K, Ikeda N, Tsuboi M, Ishii G. Prognostic impact of the tumor immune microenvironment in pulmonary pleomorphic carcinoma. Lung Cancer, 153:56-65, 2021
6. Suzuki J, Aokage K, Neri S, Sakai T, Hashimoto H, Su Y, Yamazaki S, Nakamura H, Tane K, Miyoshi T, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Tsuboi M, Ishii G. Relationship between podoplanin-expressing cancer-associated fibroblasts and the immune microenvironment of early lung squamous cell carcinoma. Lung Cancer, 153:1-10, 2021
7. Watanabe M, Kuwata T, Setsuda A, Tokunaga M, Kaito A, Sugita S, Tonouchi A, Kinoshita T, Nagino M. Molecular and pathological analyses of gastric stump cancer by next-generation sequencing and immunohistochemistry. Sci Rep, 11:4165, 2021
8. Akagi K, Oki E, Taniguchi H, Nakatani K, Aoki D, Kuwata T, Yoshino T. Real-world data on microsatellite instability status in various unresectable or metastatic solid tumors. Cancer Sci, 112:1105-1113, 2021
9. Kuroe T, Watanabe R, Kojima M, Morisue R, Sugano M, Kuwata T, Masuda H, Kusuhara S, Matsubara N, Oda S, Ushiku T, Ishii G. Evaluation of the morphological features and unfavorable prognostic impact of dirty necrosis in renal cell carcinoma. J Cancer Res Clin Oncol, 147:1089-1100, 2021
10. Mukohara T, Hosono A, Mimaki S, Nakayama A, Kusuhara S, Funasaka C, Nakao T, Fukasawa Y, Kondoh C, Harano K, Naito Y, Matsubara N, Tsuchihara K, Kuwata T. Effects of Ado-Trastuzumab Emtansine and Fam-Trastuzumab Deruxtecan on Metastatic Breast Cancer Harboring HER2 Amplification and the L755S Mutation. Oncologist, 2021
11. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y, Matsui S, Shibahara T, Yamashita Y, Irie T, Tsuge A, Fukuoka S, Kawazoe A, Udagawa H, Kirita K, Aokage K, Ishii G, Kuwata T, Nakama K, Kawazu M, Ueno T, Yamazaki N, Goto K, Tsuboi M, Mano H, Doi T, Shitara K, Nishikawa H. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol, 21:1346-1358, 2020
12. Kuwata T, Wakabayashi M, Hatanaka Y, Morii E, Oda Y, Taguchi K, Noguchi M, Ishikawa Y, Nakajima T, Sekine S, Nomura S, Okamoto W, Fujii S, Yoshino T. Impact of DNA integrity on the success rate of tissue-based next-generation sequencing: Lessons from nationwide cancer genome screening project SCRUM-Japan GI-SCREEN. Pathol Int, 70:932-942, 2020
13. Shingaki S, Kogawa T, Shimokawa M, Harano K, Naito Y, Kusuhara S, Fujimoto Y, Matsubara N, Hosono A, Mukai H, Onishi T, Hojo T, Mukohara T. Use of eribulin as an earlier-line chemotherapy for patients with HER2-negative metastatic breast cancer. J Cancer, 11:4099-4105, 2020
14. Fujimoto Y, Morita TY, Ohashi A, Haeno H, Hakozaki Y, Fujii M, Kashima Y, Kobayashi SS, Mukohara T. Combination treatment with a PI3K/Akt/mTOR pathway inhibitor overcomes resistance to anti-HER2 therapy in PIK3CA-mutant HER2-positive breast cancer cells. Sci Rep, 10:21762, 2020
15. Saito T, Makiura D, Inoue J, Doi H, Yakushijin K, Okamura A, Matsuoka H, Mukohara T, Saura R, Sakai Y, Ono R. Comparison between quantitative and subjective assessments of chemotherapy-induced peripheral neuropathy in cancer patients: A prospective cohort study. Phys Ther Res, 23:166-171, 2020
16. Sasaki A, Harano K, Kogawa T, Matsubara N, Naito Y, Hosono A, Mukai H, Yoshino T, Mukohara T. Intestinal Perforation due to Neutropenic Enterocolitis in a Patient Treated with Bevacizumab for Ovarian Cancer. Case Rep Oncol Med, 2020:7231358, 2020
17. Iwase T, Harano K, Masuda H, Kida K, Hess KR, Wang Y, Dirix L, Van Laere SJ, Lucci A, Krishnamurthy S, Woodward WA, Layman RM, Bertucci F, Ueno NT. Quantitative hormone receptor (HR) expression and gene expression analysis in HR+ inflammatory breast cancer (IBC) vs non-IBC. BMC Cancer, 20:430, 2020
18. Takehara K, Yamashita N, Watanabe R, Teramoto N, Tsuda H, Motohashi T, Harano K, Nakanishi T, Tokunaga H, Susumu N, Ueda Y, Yokoyama Y, Saito T. Clinical status and prognostic factors in Japanese patients with uterine leiomyosarcoma. Gynecol Oncol, 157:115-120, 2020
19. Inoue M, Naito Y, Kogawa T, Kusuhara S, Fukasawa Y, Fukasawa Y, Harano K Matsubara N, Hosono A, Mukohara T. Safety and Efficacy of Palbociclib in Male Metastatic Breast Cancer: A Report of Two Cases. Ann Case Report, 14:416, 2020
20. Sato K, Mimaki S, Yamashita R, Togashi Y, Naito T, Udagawa H, Katsumata S, Nakasone S, Miyoshi T, Tane K, Aokage K, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Goto K, Tsuboi M, Tsuchihara K, Ishii G. Association between the mutational smoking signature and the immune microenvironment in lung adenocarcinoma. Lung Cancer, 147:12-20, 2020
21. Koike Y, Aokage K, Ikeda K, Nakai T, Tane K, Miyoshi T, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Tanaka T, Suzuki K, Tsuboi M, Ishii G. Machine learning-based histological classification that predicts recurrence of peripheral lung squamous cell carcinoma. Lung Cancer, 147:252-258, 2020
22. Sugita S, Kinoshita T, Kuwata T, Tokunaga M, Kaito A, Watanabe M, Tonouchi A, Sato R, Nagino M. Intramucosal-lymphatic invasion has a slight impact on lymph node metastasis in patients with early gastric cancer. Surg Today, 50:484-489, 2020
23. Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, Sato E, Kuwata T, Kinoshita T, Yamamoto M, Nomura S, Tsukamoto T, Mano H, Shitara K, Nishikawa H. An Oncogenic Alteration Creates a Microenvironment that Promotes Tumor Progression by Conferring a Metabolic Advantage to Regulatory T Cells. Immunity, 53:187-203.e8, 2020
24. Sugita S, Kuwata T, Tokunaga M, Kaito A, Watanabe M, Tonouchi A, Kinoshita T, Nagino M. Clinical significance of lymphatic invasion in the esophageal region in patients with adenocarcinoma of the esophagogastric junction. J Surg Oncol, 122:433-441, 2020
25. Sato D, Takamatsu T, Umezawa M, Kitagawa Y, Maeda K, Hosokawa N, Okubo K, Kamimura M, Kadota T, Akimoto T, Kinoshita T, Yano T, Kuwata T, Ikematsu H, Takemura H, Yokota H, Soga K. Distinction of surgically resected gastrointestinal stromal tumor by near-infrared hyperspectral imaging. Sci Rep, 10:21852, 2020
26. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol, 38:2053-2061, 2020
27. Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, Mikamoto Y, Shima H, Fujishiro N, Higuchi T, Sato A, Kuwata T, Shitara K. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol, 21:1057-1065, 2020
28. Ishii T, Kawazoe A, Sasaki A, Mishima S, Kentaro S, Nakamura Y, Kotani D, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Kuwata T, Ishii G, Shitara K. Clinical and molecular factors for selection of nivolumab or irinotecan as third-line treatment for advanced gastric cancer. Ther Adv Med Oncol, 12:1758835920942377, 2020
29. Kawazoe A, Kuboki Y, Shinozaki E, Hara H, Nishina T, Komatsu Y, Yuki S, Wakabayashi M, Nomura S, Sato A, Kuwata T, Kawazu M, Mano H, Togashi Y, Nishikawa H, Yoshino T. Multicenter Phase I/II Trial of Napabucasin and Pembrolizumab in Patients with Metastatic Colorectal Cancer (EPOC1503/SCOOP Trial). Clin Cancer Res, 26:5887-5894, 2020
30. Nakamura Y, Sasaki A, Yukami H, Jogo T, Kawazoe A, Kuboki Y, Taniguchi H, Yamashita R, Kuwata T, Ozawa M, Nakamura M, Yoshino T, Shitara K. Emergence of Concurrent Multiple EGFR Mutations and MET Amplification in a Patient With EGFR-Amplified Advanced Gastric Cancer Treated With Cetuximab. JCO Precis Oncol, 4:2020
31. Tsumura R, Koga Y, Hamada A, Kuwata T, Sasaki H, Doi T, Aikawa K, Ohashi A, Katano I, Ikarashi Y, Ito M, Ochiai A. Report of the use of patient-derived xenograft models in the development of anticancer drugs in Japan. Cancer Sci, 111:3386-3394, 2020
32. Umemoto K, Togashi Y, Arai Y, Nakamura H, Takahashi S, Tanegashima T, Kato M, Nishikawa T, Sugiyama D, Kojima M, Gotohda N, Kuwata T, Ikeda M, Shibata T, Nishikawa H. The potential application of PD-1 blockade therapy for early-stage biliary tract cancer. Int Immunol, 32:273-281, 2020
33. Kaito A, Kuwata T, Tokunaga M, Shitara K, Sato R, Akimoto T, Kinoshita T. Erratum: Author's Affiliation Correction. Type II human epidermal growth factor receptor heterogeneity is a poor prognosticator for type II human epidermal growth factor receptor positive gastric cancer (World J Clin Cases 2019; Aug 6; 7 (15): 1964-1977). World J Clin Cases, 8:5494-5495, 2020
