Annual Report 2020
Department of Head and Neck Medical Oncology
Ken Kato, Yoshitaka Honma, Shun Yamamoto
Introduction
The Department of Head and Neck Medical Oncology focuses on developing new drugs and establishing standard chemotherapy regimens, including multimodality treatment with surgery and/or radiotherapy for advanced head and neck cancer (HNC), consisting of malignancies arising from the oral cavity, nasopharynx, oropharynx, hypopharynx, larynx, nasal/paranasal cavity, salivary gland, ear canal, and thyroid, etc. This year, we have focused on the multimodality treatment of esophageal cancer (EC), mainly chemotherapy and chemoradiotherapy. The main histology of HNC and EC is squamous cell carcinoma. However, there is a wide variety of histological types, especially in the nasal/paranasal cavity, salivary glands, and gastroesophageal-junctional cancer. Therefore, the pathological diagnosis is essential, making a treatment strategy based on pathological findings significant in advanced HNC and EC.
The Team and What We Do
The staff of the Department of Head and Neck Medical Oncology consists of 3 medical oncologists. We hold a daily case conference at 6 pm after routine clinical work and also hold a monthly research conference to discuss the progress of clinical trials or in-house research. Intergroup meetings with the Departments of Head and Neck Surgery, Esophageal Surgery, Endoscopy, Radiation Oncology, Diagnostic Radiology, and Diagnostic Pathology are held weekly to decide on the optimal treatment strategies for each case and to discuss treatment consensus for the disease. In 2020, we treated 476 hospitalized patients (156 of whom were newly diagnosed). Of these, 35 were enrolled in clinical trials.
Research activities
A trans-oral/percutaneous biopsy and blood sampling before and after administering cetuximab and nivolumab provide an excellent opportunity for studying biomarkers. We collect these fresh samples from patients with HNSCC to evaluate the correlations between gene expression or immunogenic profiles and patients’ outcomes by using genome sequencing, immune panels, microarrays, and real-time PCR techniques.
We also measure the gene expressions of possible predictive biomarkers by using FFPE samples obtained from surgical resection or trans-oral/percutaneous biopsy. These studies are being performed in collaboration with the Center for Medical Genomics, National Cancer Center Research Institute (NCCRI) and other institutions.
We conducted the investigator-initiated trial (FRONTiER study) for patients with locally resectable esophageal squamous cell carcinoma, which evaluated the safety and efficacy of neoadjuvant chemotherapy with nivolumab. Along with this multicenter trial, we have also conducted the biomarker analysis of immunotherapy using biopsy samples, fecal samples and other types of samples in partnership with collaborators at NCCRI and other institutions.
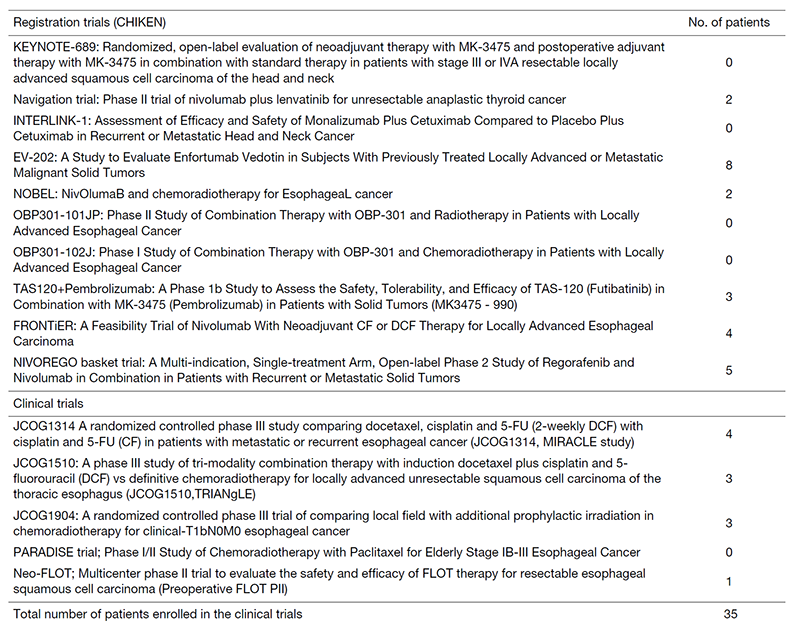
Clinical trials
We have conducted clinical trials in collaboration with the following departments in our hospital: Head and Neck Surgery, Esophageal Surgery, Gastrointestinal Endoscopy, Radiation Oncology, Diagnostic Radiology, and Diagnostic Pathology. Details of the clinical trials are summarized in the table, including JCOG (Japan Clinical Oncology Group) trials, company-initiated trials, and other collaborative investigator-initiated trials.
Education
We performed daily on-the-job training with the residents. Each staff member gives a lecture to the residents every 3 months. Each resident is given a research theme, which they develop with the staff. After analyzing the data, the residents are given the opportunity to present at medical congresses and to create a manuscript for medical journals.
Future Prospects
In April 2020, the chief of the Department of Head and Neck Medical Oncology changed from Dr. Boku to Dr. Kato. Also, esophageal cancer has become one of the focuses for our department. In May 2020, Dr. Yamamoto joined our department staff. Since 2020, our mission has been to develop new drugs and strategies using a multi-modal approach, not only for head and neck cancer but also for esophageal cancer. We have been conducting an investigator-initiated trial with nivolumab for patients with resectable esophageal cancer, in which we also conduct translational research using biopsy and blood samples to predict the efficacy of nivolumab. We plan to host a web conference with foreign investigators in Taiwan and Korea using a web conference system, which will be a good opportunity for young researchers to give presentations in English.
List of papers published in 2020
Journal
1. Hara H, Mizusawa J, Hironaka S, Kato K, Daiko H, Abe T, Nakamura K, Ando N, Kitagawa Y. Influence of preoperative chemotherapy-induced leukopenia on survival in patients with esophageal squamous cell carcinoma: exploratory analysis of JCOG9907. Esophagus, 18:41-48, 2021
2. Hasegawa H, Taniguchi H, Nakamura Y, Kato T, Fujii S, Ebi H, Shiozawa M, Yuki S, Masuishi T, Kato K, Izawa N, Moriwaki T, Oki E, Kagawa Y, Denda T, Nishina T, Tsuji A, Hara H, Esaki T, Nishida T, Kawakami H, Sakamoto Y, Miki I, Okamoto W, Yamazaki K, Yoshino T. FMS-like tyrosine kinase 3 (FLT3) amplification in patients with metastatic colorectal cancer. Cancer Sci, 112:314-322, 2021
3. Tsutsui K, Namikawa K, Mori T, Kato K, Jinnai S, Nakama K, Ogata D, Takahashi A, Yamazaki N. Case of acquired reactive perforating collagenosis induced by panitumumab for colon cancer. J Dermatol, 48:e114-e115, 2021
4. Takahashi M, Kato K, Okada M, Chin K, Kadowaki S, Hamamoto Y, Doki Y, Kubota Y, Kawakami H, Ogata T, Hara H, Muto M, Nakashima Y, Ishihara R, Tsuda M, Motoyama S, Kodani M, Kitagawa Y. Nivolumab versus chemotherapy in Japanese patients with advanced esophageal squamous cell carcinoma: a subgroup analysis of a multicenter, randomized, open-label, phase 3 trial (ATTRACTION-3). Esophagus, 18:90-99, 2021
5. Fukunaga K, Kato K, Okusaka T, Saito T, Ikeda M, Yoshida T, Zembutsu H, Iwata N, Mushiroda T. Functional Characterization of the Effects of N-acetyltransferase 2 Alleles on N-acetylation of Eight Drugs and Worldwide Distribution of Substrate-Specific Diversity. Front Genet, 12:652704, 2021
6. Iwasa S, Okita N, Kuchiba A, Ogawa G, Kawasaki M, Nakamura K, Shoji H, Honma Y, Takashima A, Kato K, Hamaguchi T, Boku N, Yamada Y. Phase II study of lenvatinib for metastatic colorectal cancer refractory to standard chemotherapy: the LEMON study (NCCH1503). ESMO Open, 5:2020
7. Shimoyama R, Hijioka S, Mizuno N, Ogawa G, Kataoka T, Katayama H, Machida N, Honma Y, Boku N, Hamaguchi T, Fukuda H, Terashima M, Kanemitsu Y, Furuse J. Study protocol for a multi-institutional randomized phase III study comparing combined everolimus plus lanreotide therapy and everolimus monotherapy in patients with unresectable or recurrent gastroenteropancreatic neuroendocrine tumors; Japan Clinical Oncology Group Study JCOG1901 (STARTER-NET study). Pancreatology, 20:1183-1188, 2020
8. Hironaka S, Komori A, Machida R, Ito Y, Takeuchi H, Ogawa G, Kato K, Onozawa M, Minashi K, Yano T, Nakamura K, Tsushima T, Hara H, Nozaki I, Ura T, Chin K, Fukuda H, Kitagawa Y. The association of primary tumor site with acute adverse event and efficacy of definitive chemoradiotherapy for cStage II/III esophageal cancer: an exploratory analysis of JCOG0909. Esophagus, 17:417-424, 2020
9. Bando H, Kotani D, Tsushima T, Hara H, Kadowaki S, Kato K, Chin K, Yamaguchi K, Kageyama SI, Hojo H, Nakamura M, Tachibana H, Wakabayashi M, Fukutani M, Togashi Y, Fuse N, Nishikawa H, Kojima T. TENERGY: multicenter phase II study of Atezolizumab monotherapy following definitive Chemoradiotherapy with 5-FU plus Cisplatin in patients with unresectable locally advanced esophageal squamous cell carcinoma. BMC Cancer, 20:336, 2020
10. Kawai S, Fukuda N, Yamamoto S, Mitani S, Omae K, Wakatsuki T, Kato K, Kadowaki S, Takahari D, Boku N, Muro K, Machida N. Retrospective observational study of salvage line ramucirumab monotherapy for patients with advanced gastric cancer. BMC Cancer, 20:338, 2020
11. Daiko H, Kato K. Updates in the 8th edition of the TNM staging system for esophagus and esophagogastric junction cancer. Jpn J Clin Oncol, 50:847-851, 2020
12. Nagata Y, Kato K, Miyamoto T, Hirano H, Shoji H, Iwasa S, Honma Y, Takashima A, Hamaguchi T, Matsushita H, Nagashima K, Saruta M, Boku N. Safety and efficacy of cell-free and concentrated ascites reinfusion therapy (CART) in gastrointestinal cancer patients with massive ascites treated with systemic chemotherapy. Support Care Cancer, 28:5861-5869, 2020
13. Iwasa S, Kudo T, Takahari D, Hara H, Kato K, Satoh T. Correction to: Practical guidance for the evaluation of disease progression and the decision to change treatment in patients with advanced gastric cancer receiving chemotherapy. Int J Clin Oncol, 25:1233, 2020
14. Yamamoto S, Kato K, Daiko H, Kojima T, Hara H, Abe T, Tsubosa Y, Nagashima K, Aoki K, Mizoguchi Y, Kitano S, Yachida S, Shiba S, Kitagawa Y. Feasibility study of nivolumab as neoadjuvant chemotherapy for locally esophageal carcinoma: FRONTiER (JCOG1804E). Future Oncol, 16:1351-1357, 2020
15. Hashimoto H, Iwasa S, Yanai-Takahashi T, Honma Y, Kato K, Hamaguchi T, Yamada Y, Shimada Y, Yamazaki N, Kato Y. Randomized, Double-Blind, Placebo-Controlled Phase II Study on the Efficacy and Safety of Vitamin K1 Ointment for Cetuximab or Panitumumab-Induced Acneiform Eruptions-VIKTORIA Study. Gan To Kagaku Ryoho, 47:933-939, 2020
16. Nakatani Y, Kato K, Shoji H, Iwasa S, Honma Y, Takashima A, Ushijima T, Ito Y, Itami J, Boku N. Comparison of involved field radiotherapy and elective nodal irradiation in combination with concurrent chemotherapy for T1bN0M0 esophageal cancer. Int J Clin Oncol, 25:1098-1104, 2020
17. Kato K, Doki Y, Ura T, Hamamoto Y, Kojima T, Tsushima T, Hironaka S, Hara H, Kudo T, Iwasa S, Muro K, Yasui H, Minashi K, Yamaguchi K, Ohtsu A, Kitagawa Y. Long-term efficacy and predictive correlates of response to nivolumab in Japanese patients with esophageal cancer. Cancer Sci, 111:1676-1684, 2020
18. Daiko H, Marafioti T, Fujiwara T, Shirakawa Y, Nakatsura T, Kato K, Puccio I, Hikichi T, Yoshimura S, Nakagawa T, Furukawa M, Stoeber K, Nagira M, Ide N, Kojima T. Exploratory open-label clinical study to determine the S-588410 cancer peptide vaccine-induced tumor-infiltrating lymphocytes and changes in the tumor microenvironment in esophageal cancer patients. Cancer Immunol Immunother, 69:2247-2257, 2020
19. Yamamoto S, Kato K. Pembrolizumab for the treatment of esophageal cancer. Expert Opin Biol Ther, 20:1143-1150, 2020
20. Imazeki H, Kato K. Development of chemotherapeutics for unresectable advanced esophageal cancer. Expert Rev Anticancer Ther, 20:1083-1092, 2020
21. Nakamura Y, Taniguchi H, Ikeda M, Bando H, Kato K, Morizane C, Esaki T, Komatsu Y, Kawamoto Y, Takahashi N, Ueno M, Kagawa Y, Nishina T, Kato T, Yamamoto Y, Furuse J, Denda T, Kawakami H, Oki E, Nakajima T, Nishida N, Yamaguchi K, Yasui H, Goto M, Matsuhashi N, Ohtsubo K, Yamazaki K, Tsuji A, Okamoto W, Tsuchihara K, Yamanaka T, Miki I, Sakamoto Y, Ichiki H, Hata M, Yamashita R, Ohtsu A, Odegaard JI, Yoshino T. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med, 26:1859-1864, 2020
22. Yamamoto S, Kato K. Immuno-oncology for esophageal cancer. Future Oncol, 16:2673-2681, 2020
23. Tamura N, Honma Y, Sekine S, Tsukamoto S, Hirano H, Okita N, Shoji H, Iwasa S, Takashima A, Kato K, Boku N. Case report: potential treatment of metastatic amphicrine carcinoma of the rectum with FOLFOXIRI chemotherapy. Oxf Med Case Reports, 2020:omaa097, 2020
24. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, Bennouna J, Kato K, Shen L, Enzinger P, Qin SK, Ferreira P, Chen J, Girotto G, de la Fouchardiere C, Senellart H, Al-Rajabi R, Lordick F, Wang R, Suryawanshi S, Bhagia P, Kang SP, Metges JP. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol, 38:4138-4148, 2020
25. Chen LT, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Sameshima H, Kang YK, Boku N. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer, 23:510-519, 2020
26. Otsuka R, Iwasa S, Yanai T, Hirano H, Shoji H, Honma Y, Okita N, Takashima A, Kato K, Hashimoto H, Sekiguchi M, Makino Y, Boku N, Yamaguchi M. Impact of peripheral neuropathy induced by platinum in first-line chemotherapy on second-line chemotherapy with paclitaxel for advanced gastric cancer. Int J Clin Oncol, 25:595-601, 2020
27. Okada M, Yamamoto S, Kato K. Nivolumab for the Treatment of Esophageal Squamous Cell Carcinoma. Oncology & Hematology Review, 16:90-94, 2020
28. Ito T, Fujimori N, Honma Y, Kudo A, Hijioka S, Katsushima S, Kimura Y, Fukutomi A, Hisamatsu S, Nakajima A, Shimatsu A. Long-term safety and efficacy of lanreotide autogel in Japanese patients with neuroendocrine tumors: Final results of a phase II open-label extension study. Asia Pac J Clin Oncol, 2020
29. Miyamoto T, Kato K. Immunotherapy for esophageal carcinoma: a narrative review. Shanghai Chest, doi: 10.21037/shc-20-69, 2020
