Annual Report 2020
Department of Gastrointestinal Medical Oncology
Narikazu Boku, Ken Kato, Atsuo Takashima, Satoru Iwasa, Yoshitaka Honma, Hirokazu Shoji, Natsuko Okita, Hidekazu Hirano, Shun Yamamoto, Kotoe Oshima
Introduction
Department of Gastrointestinal Medical Oncology focuses not only on the treatment in clinical practice but also on the development of new drugs and establishment of new standard therapies, including multi-modality treatment with surgery and/or radiotherapy for gastric/colorectal/gastrointestinal stromal tumor (GIST), and other gastrointestinal (GI) malignancies.
The Team and What We Do
The staff of our division consists of 9 medical oncologists, 1 chief resident, and 3 or 4 rotating residents. We have a daily case conference for discussing each patient’s treatment every evening and a weekly research conference for sharing and discussing the progress of clinical and/or translational research. Multidisciplinary team meetings with the surgical division (Colorectal, and Gastric Surgery Divisions) and the Radiation Oncology Division are held weekly to decide optimal treatment for each patient and to discuss the common treatment strategy. We managed 25.6 hospitalized patients/day (total 9,360/year) and 40.8 outpatients/day (total 14,889/year), including 451 newly diagnosed patients in 2020.
Research activities
We are making great efforts to conduct various clinical studies to establish new treatments and answer clinical questions. As for late-phase clinical studies, our division is playing a leading role in JCOG (Japan Clinical Oncology Group) and WJOG (West Japan Oncology Group), both of which are the largest cooperative study groups in Japan. For early phase clinical studies, in collaboration with other active hospitals, we are conducting investigator-initiated trials using unapproved drugs under the regulation of GCP (Good Clinical Practice). Moreover, we are collaborating with the National Cancer Center Research Institute and other distinguished basic research institutions to perform clinical research based on our own ideas and findings. We published 56 English articles in 2020.
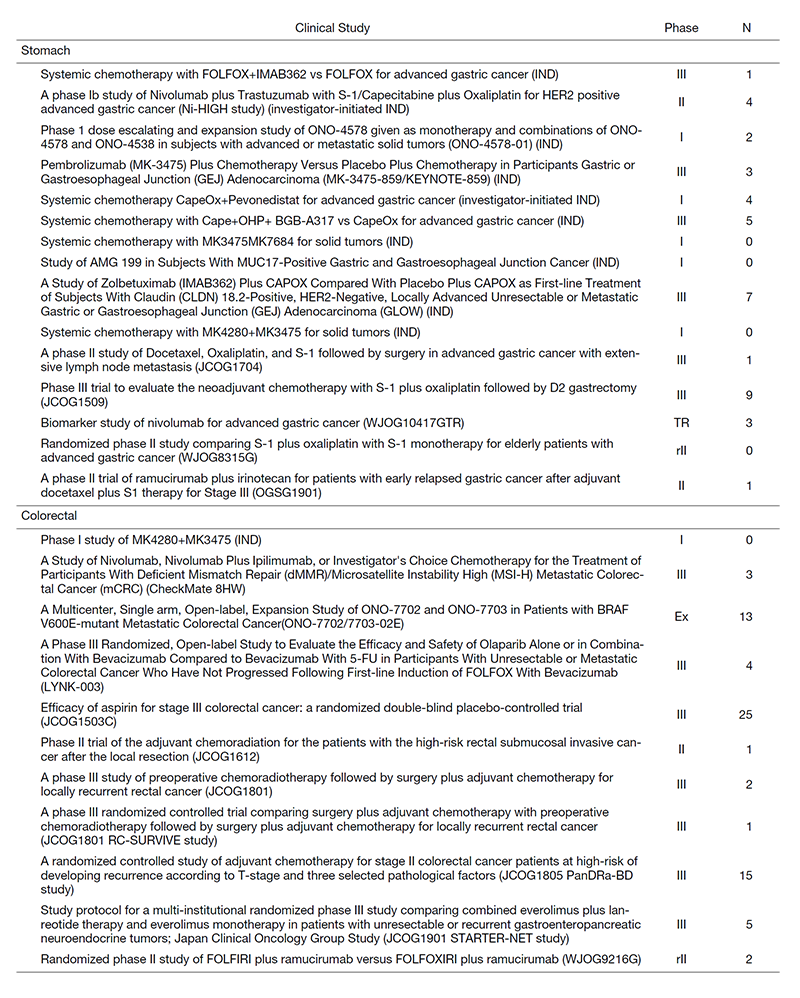
Clinical trials
We are conducting many clinical trials, including JCOG, WJOG, company-initiated trials, and other collaborative investigator-initiated trials and in-house clinical trials, to which a total of 111 patients were enrolled in 2020 (Table 1).
Education
The chief resident can learn basic research methods in collaboration with the National Cancer Center Research Institute.
Future Prospects
Our division focus on “clinical practice”, “education” and “clinical research” for the development of gastrointestinal cancer treatment. For daily practice, we provide patients with the standard treatment; however, we will always remain alert to discovering something more effective for the patient’s care to satisfy their unmet clinical needs. Regarding education, we will continue educating the residents, chief residents, and the staff doctors to keep improving. For clinical research, we will take the next step of clinical development to establish new global standard treatments.
List of papers published in 2020
Journal
1. Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito S, Kaji M, Kimura Y, Hirao M, Yamada M, Kurita A, Takagi M, Lee SW, Takagane A, Yabusaki H, Hihara J, Boku N, Sano T, Sasako M. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer, 24:492-502, 2021
2. Fujitani K, Nakamura K, Mizusawa J, Kuwata T, Shimoda T, Katayama H, Kushima R, Taniguchi H, Yoshikawa T, Boku N, Terashima M, Fukuda H, Sano T, Sasako M. Posttherapy topographical nodal status, ypN-site, predicts survival of patients who received neoadjuvant chemotherapy followed by curative surgical resection for non-type 4 locally advanced gastric cancer: supplementary analysis of JCOG1004-A. Gastric Cancer, 24:197-204, 2021
3. Ego M, Abe S, Nakatani Y, Nonaka S, Suzuki H, Yoshinaga S, Oda I, Kato K, Honma Y, Itami J, Daiko H, Saito Y, Boku N. Long-term outcomes of patients with recurrent squamous cell carcinoma of the esophagus undergoing salvage endoscopic resection after definitive chemoradiotherapy. Surg Endosc, 35:1766-1776, 2021
4. Fukunaga K, Hishinuma E, Hiratsuka M, Kato K, Okusaka T, Saito T, Ikeda M, Yoshida T, Zembutsu H, Iwata N, Mushiroda T. Determination of novel CYP2D6 haplotype using the targeted sequencing followed by the long-read sequencing and the functional characterization in the Japanese population. J Hum Genet, 66:139-149, 2021
5. Narita Y, Shoji H, Kawai S, Mizukami T, Nakamura M, Moriwaki T, Yamanaka T, Sunakawa Y, Kawakami H, Nishina T, Misumi T, Muro K. REVIVE study: a prospective observational study in chemotherapy after nivolumab therapy for advanced gastric cancer. Future Oncol, 17:869-875, 2021
6. Aogi K, Takeuchi H, Saeki T, Aiba K, Tamura K, Iino K, Imamura CK, Okita K, Kagami Y, Tanaka R, Nakagawa K, Fujii H, Boku N, Wada M, Akechi T, Iihara H, Ohtani S, Okuyama A, Ozawa K, Kim YI, Sasaki H, Shima Y, Takeda M, Nagasaki E, Nishidate T, Higashi T, Hirata K. Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan: Update summary of the 2015 Japan Society of Clinical Oncology Clinical Practice Guidelines for Antiemesis. Int J Clin Oncol, 26:1-17, 2021
7. Moehler M, Dvorkin M, Boku N, Özgüroğlu M, Ryu MH, Muntean AS, Lonardi S, Nechaeva M, Bragagnoli AC, Coşkun HS, Cubillo Gracian A, Takano T, Wong R, Safran H, Vaccaro GM, Wainberg ZA, Silver MR, Xiong H, Hong J, Taieb J, Bang YJ. Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100. J Clin Oncol, 39:966-977, 2021
8. Hirano H, Takashima A, Hamaguchi T, Shida D, Kanemitsu Y. Current status and perspectives of immune checkpoint inhibitors for colorectal cancer. Jpn J Clin Oncol, 51:10-19, 2021
9. Takeuchi M, Imamura CK, Booka E, Takeuchi H, Mizukami T, Kawakami T, Funakoshi T, Wakuda K, Aoki Y, Hamamoto Y, Kitago M, Kawakubo H, Boku N, Tanigawara Y, Kitagawa Y. Prospective evaluation and refinement of an S-1 dosage formula based on renal function for clinical application. Cancer Sci, 112:751-759, 2021
10. Hara H, Mizusawa J, Hironaka S, Kato K, Daiko H, Abe T, Nakamura K, Ando N, Kitagawa Y. Influence of preoperative chemotherapy-induced leukopenia on survival in patients with esophageal squamous cell carcinoma: exploratory analysis of JCOG9907. Esophagus, 18:41-48, 2021
11. Hasegawa H, Taniguchi H, Nakamura Y, Kato T, Fujii S, Ebi H, Shiozawa M, Yuki S, Masuishi T, Kato K, Izawa N, Moriwaki T, Oki E, Kagawa Y, Denda T, Nishina T, Tsuji A, Hara H, Esaki T, Nishida T, Kawakami H, Sakamoto Y, Miki I, Okamoto W, Yamazaki K, Yoshino T. FMS-like tyrosine kinase 3 (FLT3) amplification in patients with metastatic colorectal cancer. Cancer Sci, 112:314-322, 2021
12. Tsutsui K, Namikawa K, Mori T, Kato K, Jinnai S, Nakama K, Ogata D, Takahashi A, Yamazaki N. Case of acquired reactive perforating collagenosis induced by panitumumab for colon cancer. J Dermatol, 48:e114-e115, 2021
13. Takahashi M, Kato K, Okada M, Chin K, Kadowaki S, Hamamoto Y, Doki Y, Kubota Y, Kawakami H, Ogata T, Hara H, Muto M, Nakashima Y, Ishihara R, Tsuda M, Motoyama S, Kodani M, Kitagawa Y. Nivolumab versus chemotherapy in Japanese patients with advanced esophageal squamous cell carcinoma: a subgroup analysis of a multicenter, randomized, open-label, phase 3 trial (ATTRACTION-3). Esophagus, 18:90-99, 2021
14. Shah MA, Bennouna J, Doi T, Shen L, Kato K, Adenis A, Mamon HJ, Moehler M, Fu X, Cho BC, Bordia S, Bhagia P, Shih CS, Desai A, Enzinger P. KEYNOTE-975 study design: a Phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Future Oncol, 17:1143-1153, 2021
15. Fukahori M, Kato K, Taniguchi H, Ohtomo R, Takahashi N, Shoji H, Iwasa S, Honma Y, Takashima A, Hamaguchi T, Yamada Y, Shimada Y, Ito Y, Itami J, Hokamura N, Igaki H, Tachimori Y, Miwa K, Torimura T, Boku N. Relationship between cervical esophageal squamous cell carcinoma and human papilloma virus infection and gene mutations. Mol Clin Oncol, 14:41, 2021
16. Fujitani K, Shitara K, Takashima A, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Kimura Y, Amagai K, Hosaka H, Komatsu Y, Shimada K, Kawabata R, Ohdan H, Kodera Y, Nakamura M, Nakajima TE, Miyata Y, Moriwaki T, Kusumoto T, Nishikawa K, Ogata K, Shimura M, Morita S, Koizumi W. Correction to: Effect of early tumor response on the health-related quality of life among patients on second-line chemotherapy for advanced gastric cancer in the ABSOLUTE trial. Gastric Cancer, 24:477-478, 2021
17. Takizawa K, Ono H, Hasuike N, Takashima A, Minashi K, Boku N, Kushima R, Katayama H, Ogawa G, Fukuda H, Fujisaki J, Oda I, Yano T, Hori S, Doyama H, Hirasawa K, Yamamoto Y, Ishihara R, Tanabe S, Niwa Y, Nakagawa M, Terashima M, Muto M. A nonrandomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010). Gastric Cancer, 24:479-491, 2021
18. Watanabe J, Sasaki S, Kusumoto T, Sakamoto Y, Yoshida K, Tomita N, Maeda A, Teshima J, Yokota M, Tanaka C, Yamauchi J, Uetake H, Itabashi M, Takahashi K, Baba H, Kotake K, Boku N, Aiba K, Morita S, Takenaka N, Sugihara K. S-1 and oxaliplatin versus tegafur-uracil and leucovorin as post-operative adjuvant chemotherapy in patients with high-risk stage III colon cancer: updated 5-year survival of the phase III ACTS-CC 02 trial. ESMO Open, 6:100077, 2021
19. Kawakami H, Hironaka S, Esaki T, Chayama K, Tsuda M, Sugimoto N, Kadowaki S, Makiyama A, Machida N, Hirano H, Hirata K, Hara H, Yabusaki H, Komatsu Y, Muro K. An Investigator-Initiated Phase 2 Study of Nivolumab Plus Low-Dose Ipilimumab as First-Line Therapy for Microsatellite Instability-High Advanced Gastric or Esophagogastric Junction Cancer (NO LIMIT, WJOG13320G/CA209-7W7). Cancers (Basel), 13:2021
20. Takamizawa S, Shoji H, Hirano H, Izutsu K, Yamamoto S, Iwasa S, Honma Y, Okita N, Takashima A, Kato K, Boku N. Panitumumab-Associated Drug-Induced Immune Thrombocytopenia in a Patient with Colorectal Cancer. Case Rep Oncol, 14:85-89, 2021
21. Kanamori K, Yamagata Y, Honma Y, Date K, Wada T, Hayashi T, Otsuki S, Sekine S, Yoshikawa T, Katai H, Nishida T. Extra-gastrointestinal stromal tumor arising in the lesser omentum with a platelet-derived growth factor receptor alpha (PDGFRA) mutation: a case report and literature review. World J Surg Oncol, 18:183, 2020
22. Sato Y, Mizusawa J, Katayama H, Nakamura K, Fukagawa T, Katai H, Haruta S, Yamada M, Takagi M, Tamura S, Yoshimura T, Tokunaga M, Yoshikawa T, Boku N, Sano T, Sasako M, Terashima M. Diagnosis of invasion depth in resectable advanced gastric cancer for neoadjuvant chemotherapy: An exploratory analysis of Japan clinical oncology group study: JCOG1302A. Eur J Surg Oncol, 46:1074-1079, 2020
23. Terashima M, Yoshikawa T, Boku N, Ito S, Tsuburaya A, Iwasaki Y, Fukagawa T, Tokunaga M, Sano T, Sasako M. Current status of perioperative chemotherapy for locally advanced gastric cancer and JCOG perspectives. Jpn J Clin Oncol, 50:528-534, 2020
24. Chen LT, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Sameshima H, Kang YK, Boku N. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer, 23:510-519, 2020
25. Takamizawa Y, Shida D, Boku N, Nakamura Y, Ahiko Y, Yoshida T, Tanabe T, Takashima A, Kanemitsu Y. Nutritional and inflammatory measures predict survival of patients with stage IV colorectal cancer. BMC Cancer, 20:1092, 2020
26. Otsuka R, Iwasa S, Yanai T, Hirano H, Shoji H, Honma Y, Okita N, Takashima A, Kato K, Hashimoto H, Sekiguchi M, Makino Y, Boku N, Yamaguchi M. Impact of peripheral neuropathy induced by platinum in first-line chemotherapy on second-line chemotherapy with paclitaxel for advanced gastric cancer. Int J Clin Oncol, 25:595-601, 2020
27. Nagata Y, Kato K, Miyamoto T, Hirano H, Shoji H, Iwasa S, Honma Y, Takashima A, Hamaguchi T, Matsushita H, Nagashima K, Saruta M, Boku N. Safety and efficacy of cell-free and concentrated ascites reinfusion therapy (CART) in gastrointestinal cancer patients with massive ascites treated with systemic chemotherapy. Support Care Cancer, 28:5861-5869, 2020
28. Bando H, Kotani D, Tsushima T, Hara H, Kadowaki S, Kato K, Chin K, Yamaguchi K, Kageyama SI, Hojo H, Nakamura M, Tachibana H, Wakabayashi M, Fukutani M, Togashi Y, Fuse N, Nishikawa H, Kojima T. TENERGY: multicenter phase II study of Atezolizumab monotherapy following definitive Chemoradiotherapy with 5-FU plus Cisplatin in patients with unresectable locally advanced esophageal squamous cell carcinoma. BMC Cancer, 20:336, 2020
29. Kawai S, Fukuda N, Yamamoto S, Mitani S, Omae K, Wakatsuki T, Kato K, Kadowaki S, Takahari D, Boku N, Muro K, Machida N. Retrospective observational study of salvage line ramucirumab monotherapy for patients with advanced gastric cancer. BMC Cancer, 20:338, 2020
30. Hironaka S, Komori A, Machida R, Ito Y, Takeuchi H, Ogawa G, Kato K, Onozawa M, Minashi K, Yano T, Nakamura K, Tsushima T, Hara H, Nozaki I, Ura T, Chin K, Fukuda H, Kitagawa Y. The association of primary tumor site with acute adverse event and efficacy of definitive chemoradiotherapy for cStage II/III esophageal cancer: an exploratory analysis of JCOG0909. Esophagus, 17:417-424, 2020
31. Ouchi A, Shida D, Hamaguchi T, Takashima A, Ito Y, Ueno H, Ishiguro M, Takii Y, Ikeda S, Ohue M, Fujita S, Shiozawa M, Kataoka K, Ito M, Tsukada Y, Akagi T, Inomata M, Shimada Y, Kanemitsu Y. Challenges of improving treatment outcomes for colorectal and anal cancers in Japan: the Colorectal Cancer Study Group (CCSG) of the Japan Clinical Oncology Group (JCOG). Jpn J Clin Oncol, 50:368-378, 2020
32. Moriwaki T, Fukuoka S, Masuishi T, Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Esaki T, Makiyama A, Denda T, Hatachi Y, Suto T, Sugimoto N, Enomoto M, Ishikawa T, Kashiwada T, Oki E, Komatsu Y, Tsuji A, Tsuchihashi K, Sakai D, Ueno H, Tamura T, Yamashita K, Shimada Y. Prognostic scores for evaluating the survival benefit of regorafenib or trifluridine/tipiracil in patients with metastatic colorectal cancer: an exploratory analysis of the REGOTAS study. Int J Clin Oncol, 25:614-621, 2020
33. Kato K, Doki Y, Ura T, Hamamoto Y, Kojima T, Tsushima T, Hironaka S, Hara H, Kudo T, Iwasa S, Muro K, Yasui H, Minashi K, Yamaguchi K, Ohtsu A, Kitagawa Y. Long-term efficacy and predictive correlates of response to nivolumab in Japanese patients with esophageal cancer. Cancer Sci, 111:1676-1684, 2020
34. Iwasa S, Kudo T, Takahari D, Hara H, Kato K, Satoh T. Correction to: Practical guidance for the evaluation of disease progression and the decision to change treatment in patients with advanced gastric cancer receiving chemotherapy. Int J Clin Oncol, 25:1233, 2020
35. Yamamoto S, Kato K, Daiko H, Kojima T, Hara H, Abe T, Tsubosa Y, Nagashima K, Aoki K, Mizoguchi Y, Kitano S, Yachida S, Shiba S, Kitagawa Y. Feasibility study of nivolumab as neoadjuvant chemotherapy for locally esophageal carcinoma: FRONTiER (JCOG1804E). Future Oncol, 16:1351-1357, 2020
36. Aoki M, Shoji H, Kashiro A, Takeuchi K, Shimizu Y, Honda K. Prospects for Comprehensive Analyses of Circulating Tumor Cells in Tumor Biology. Cancers (Basel), 12:2020
37. Ito T, Fujimori N, Honma Y, Kudo A, Hijioka S, Katsushima S, Kimura Y, Fukutomi A, Hisamatsu S, Nakajima A, Shimatsu A. Long-term safety and efficacy of lanreotide autogel in Japanese patients with neuroendocrine tumors: Final results of a phase II open-label extension study. Asia Pac J Clin Oncol, 2020
38. Cohen R, Vernerey D, Bellera C, Meurisse A, Henriques J, Paoletti X, Rousseau B, Alberts S, Aparicio T, Boukovinas I, Gill S, Goldberg RM, Grothey A, Hamaguchi T, Iveson T, Kerr R, Labianca R, Lonardi S, Meyerhardt J, Paul J, Punt CJA, Saltz L, Saunders MP, Schmoll HJ, Shah M, Sobrero A, Souglakos I, Taieb J, Takashima A, Wagner AD, Ychou M, Bonnetain F, Gourgou S, Yoshino T, Yothers G, de Gramont A, Shi Q, Andr? T. Guidelines for time-to-event end-point definitions in adjuvant randomised trials for patients with localised colon cancer: Results of the DATECAN initiative. Eur J Cancer, 130:63-71, 2020
39. Makiyama A, Sukawa Y, Kashiwada T, Kawada J, Hosokawa A, Horie Y, Tsuji A, Moriwaki T, Tanioka H, Shinozaki K, Uchino K, Yasui H, Tsukuda H, Nishikawa K, Ishida H, Yamanaka T, Yamazaki K, Hironaka S, Esaki T, Boku N, Hyodo I, Muro K. Randomized, Phase II Study of Trastuzumab Beyond Progression in Patients With HER2-Positive Advanced Gastric or Gastroesophageal Junction Cancer: WJOG7112G (T-ACT Study). J Clin Oncol, 38:1919-1927, 2020
40. Nakatani Y, Kato K, Shoji H, Iwasa S, Honma Y, Takashima A, Ushijima T, Ito Y, Itami J, Boku N. Comparison of involved field radiotherapy and elective nodal irradiation in combination with concurrent chemotherapy for T1bN0M0 esophageal cancer. Int J Clin Oncol, 25:1098-1104, 2020
41. Daiko H, Marafioti T, Fujiwara T, Shirakawa Y, Nakatsura T, Kato K, Puccio I, Hikichi T, Yoshimura S, Nakagawa T, Furukawa M, Stoeber K, Nagira M, Ide N, Kojima T. Exploratory open-label clinical study to determine the S-588410 cancer peptide vaccine-induced tumor-infiltrating lymphocytes and changes in the tumor microenvironment in esophageal cancer patients. Cancer Immunol Immunother, 69:2247-2257, 2020
42. Sakai D, Taniguchi H, Sugimoto N, Tamura T, Nishina T, Hara H, Esaki T, Denda T, Sakamoto T, Okuda H, Satoh T, Tsushima T, Makiyama A, Tsuda T, Hosokawa A, Kuramochi H, Tokunaga S, Moriwaki T, Yasui H, Ishida H, Tsuji A, Otsu S, Shimokawa H, Baba E, Sato M, Matsumoto S, Ozaki Y, Shinozaki K, Tamagawa H, Goto M, Kadowaki S, Fujii H, Koh Y, Yamazaki K, Hironaka S, Kishimoto J, Boku N, Hyodo I, Muro K. Randomised phase II study of panitumumab plus irinotecan versus cetuximab plus irinotecan in patients with KRAS wild-type metastatic colorectal cancer refractory to fluoropyrimidine, irinotecan and oxaliplatin (WJOG 6510G). Eur J Cancer, 135:11-21, 2020
43. Levy A, Wagner AD, Chargari C, Moehler M, Verheij M, Durand-Labrunie J, Kissel M, Chirat E, Burtin P, Ducreux M, Boige V, Nilsson M, Boku N, Chau I, Deutsch E. Palliation of dysphagia in metastatic oesogastric cancers: An international multidisciplinary position. Eur J Cancer, 135:103-112, 2020
44. Daiko H, Kato K. Updates in the 8th edition of the TNM staging system for esophagus and esophagogastric junction cancer. Jpn J Clin Oncol, 50:847-851, 2020
45. Hashimoto H, Iwasa S, Yanai-Takahashi T, Honma Y, Kato K, Hamaguchi T, Yamada Y, Shimada Y, Yamazaki N, Kato Y. Randomized, Double-Blind, Placebo-Controlled Phase II Study on the Efficacy and Safety of Vitamin K1 Ointment for Cetuximab or Panitumumab-Induced Acneiform Eruptions-VIKTORIA Study. Gan To Kagaku Ryoho, 47:933-939, 2020
46. Nakajima TE, Yamaguchi K, Boku N, Hyodo I, Mizusawa J, Hara H, Nishina T, Sakamoto T, Shitara K, Shinozaki K, Katayama H, Nakamura S, Muro K, Terashima M. Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer, 23:677-688, 2020
47. Yamamoto S, Kato K. Pembrolizumab for the treatment of esophageal cancer. Expert Opin Biol Ther, 20:1143-1150, 2020
48. Kobayashi S, Takahashi S, Takahashi N, Masuishi T, Shoji H, Shinozaki E, Yamaguchi T, Kojima M, Gotohda N, Nomura S, Yoshino T, Taniguchi H. Survival Outcomes of Resected BRAF V600E Mutant Colorectal Liver Metastases: A Multicenter Retrospective Cohort Study in Japan. Ann Surg Oncol, 27:3307-3315, 2020
49. Sato Y, Yamada T, Yoshikawa T, Machida R, Mizusawa J, Katayama H, Tokunaga M, Boku N, Terashima M. Randomized controlled Phase III trial to evaluate omentum preserving gastrectomy for patients with advanced gastric cancer (JCOG1711, ROAD-GC). Jpn J Clin Oncol, 50:1321-1324, 2020
50. Kang YK, Chin K, Chung HC, Kadowaki S, Oh SC, Nakayama N, Lee KW, Hara H, Chung IJ, Tsuda M, Park SH, Hosaka H, Hironaka S, Miyata Y, Ryu MH, Baba H, Hyodo I, Bang YJ, Boku N. S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin as first-line therapy in patients with advanced gastric cancer (SOLAR): a randomised, open-label, phase 3 trial. Lancet Oncol, 21:1045-1056, 2020
51. Iwasa S, Okita N, Kuchiba A, Ogawa G, Kawasaki M, Nakamura K, Shoji H, Honma Y, Takashima A, Kato K, Hamaguchi T, Boku N, Yamada Y. Phase II study of lenvatinib for metastatic colorectal cancer refractory to standard chemotherapy: the LEMON study (NCCH1503). ESMO Open, 5:2020
52. Yamamoto S, Kato K. Immuno-oncology for esophageal cancer. Future Oncol, 16:2673-2681, 2020
53. Imazeki H, Kato K. Development of chemotherapeutics for unresectable advanced esophageal cancer. Expert Rev Anticancer Ther, 20:1083-1092, 2020
54. Shimoyama R, Hijioka S, Mizuno N, Ogawa G, Kataoka T, Katayama H, Machida N, Honma Y, Boku N, Hamaguchi T, Fukuda H, Terashima M, Kanemitsu Y, Furuse J. Study protocol for a multi-institutional randomized phase III study comparing combined everolimus plus lanreotide therapy and everolimus monotherapy in patients with unresectable or recurrent gastroenteropancreatic neuroendocrine tumors; Japan Clinical Oncology Group Study JCOG1901 (STARTER-NET study). Pancreatology, 20:1183-1188, 2020
55. Tamura N, Honma Y, Sekine S, Tsukamoto S, Hirano H, Okita N, Shoji H, Iwasa S, Takashima A, Kato K, Boku N. Case report: potential treatment of metastatic amphicrine carcinoma of the rectum with FOLFOXIRI chemotherapy. Oxf Med Case Reports, 2020:omaa097, 2020
56. Doi T, Boku N, Onozawa Y, Takahashi K, Kawaguchi O, Ohtsu A. Phase I dose-escalation study of the safety, tolerability, and pharmacokinetics of aflibercept in combination with S-1 in Japanese patients with advanced solid malignancies. Invest New Drugs, 38:1390-1399, 2020
57. Nakamura Y, Taniguchi H, Ikeda M, Bando H, Kato K, Morizane C, Esaki T, Komatsu Y, Kawamoto Y, Takahashi N, Ueno M, Kagawa Y, Nishina T, Kato T, Yamamoto Y, Furuse J, Denda T, Kawakami H, Oki E, Nakajima T, Nishida N, Yamaguchi K, Yasui H, Goto M, Matsuhashi N, Ohtsubo K, Yamazaki K, Tsuji A, Okamoto W, Tsuchihara K, Yamanaka T, Miki I, Sakamoto Y, Ichiki H, Hata M, Yamashita R, Ohtsu A, Odegaard JI, Yoshino T. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med, 26:1859-1864, 2020
58. Zenda S, Ryu A, Takashima A, Arai M, Takagi Y, Miyaji T, Mashiko T, Shimizu Y, Yamazaki N, Morizane C, Yamaguchi T, Kawaguchi T, Hanai A, Uchitomi Y, Oshiba F. Hydrocolloid dressing as a prophylactic use for hand-foot skin reaction induced by multitargeted kinase inhibitors: protocol of a phase 3 randomised self-controlled study. BMJ Open, 10:e038276, 2020
59. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, Bennouna J, Kato K, Shen L, Enzinger P, Qin SK, Ferreira P, Chen J, Girotto G, de la Fouchardiere C, Senellart H, Al-Rajabi R, Lordick F, Wang R, Suryawanshi S, Bhagia P, Kang SP, Metges JP. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol, 38:4138-4148, 2020
60. Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, Saito K, Kawaguchi Y, Kamio T, Kojima A, Sugihara M, Yamaguchi K. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med, 382:2419-2430, 2020
