Annual Report 2020
Department of Hepatobiliary and Pancreatic Oncology
Takuji Okusaka, Hideki Ueno, Chigusa Morizane, Susumu Hijioka, Shunsuke Kondo, Akihiro Ohba, Yoshikuni Nagashio, Yuta Maruki, Yuya Hisada, Motohiro Yoshinari
Introduction
The Department of Hepatobiliary and Pancreatic Oncology treats tumors originating from the liver, biliary system, and pancreas, which include hepatocellular carcinoma (HCC), biliary tract cancer, and pancreatic cancer. As part of the multidisciplinary care given at the National Cancer Center Hospital (NCCH), we work closely with surgeons and radiologists who have special expertise in these areas. We also conduct clinical and translational researches for hepatobiliary and pancreatic tumors and seek to develop new and more effective diagnostic methods and treatments.
The Team and What We Do
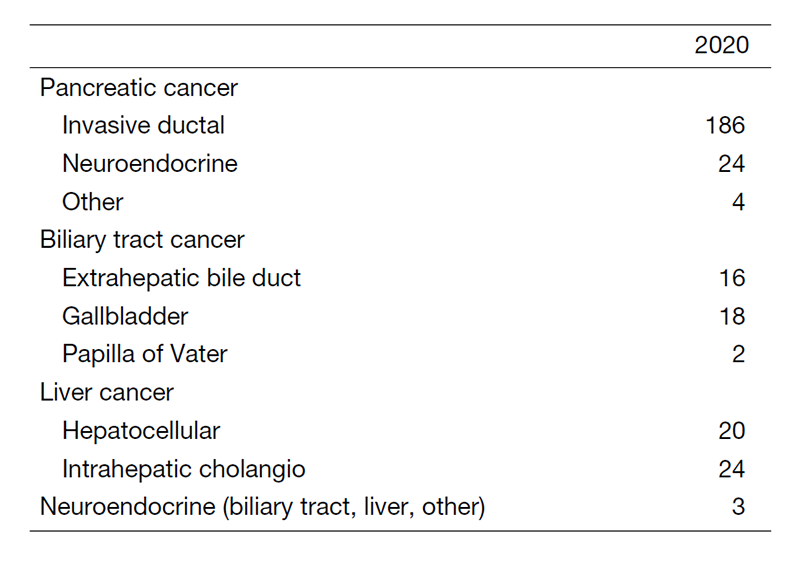
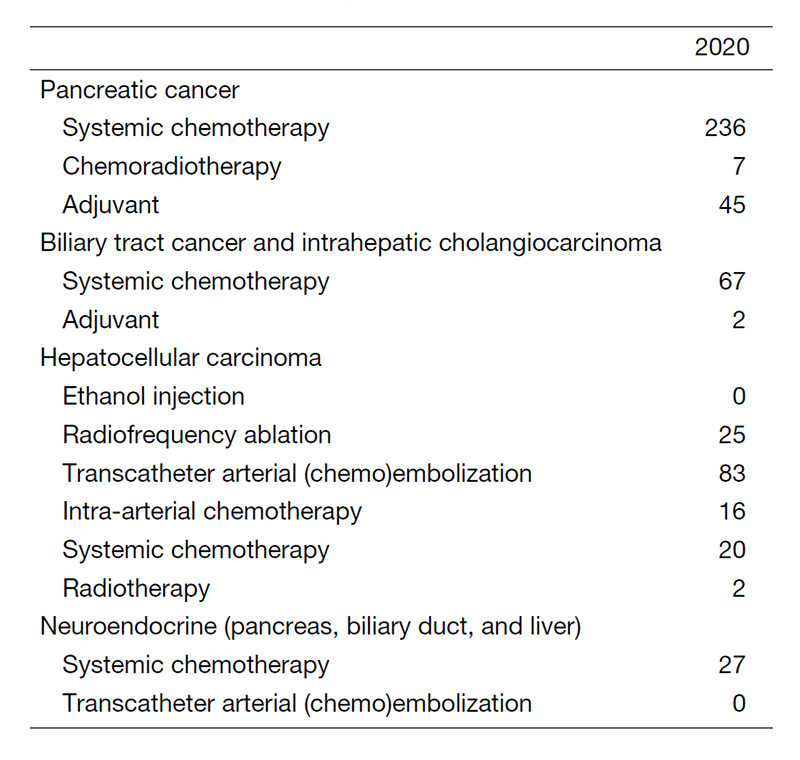
The department consists of seven staff oncologists and several residents. We have used percutaneous ablation therapy for most patients with three or fewer HCC nodules, all of which are smaller than 3 cm in diameter. We also perform transcatheter arterial chemoembolization (TACE), mainly in patients with multiple HCC nodules. Systemic or intra-arterial chemotherapeutic regimens are indicated in advanced HCC patients for whom locoregional intervention and surgery are unsuitable or had been unsuccessful. In patients with unresectable pancreatic cancer or biliary tract cancer, chemotherapy is performed in clinical practice or as a clinical trial to develop new treatment. We have actively introduced endoscopic procedures for imaging diagnosis (endoscopic ultrasonography: EUS, endoscopic retrograde cholangiopancreatography: ERCP), tumor biopsy (EUS-guided fine needle aspiration: EUS-FNA), and biliary drainage including EUS-guided hepaticogastrostomy (EUS-HGS) and EUS-guided choledochoduodenostomy (EUS-CDS) (Tables 1 & 2).
Research activities
We published 10 papers as a first author in peer-reviewed journals in 2020.
Gemcitabine plus cisplatin (GC) is the standard treatment of advanced biliary tract cancer (BTC). Gemcitabine plus S-1 (GS) reportedly has equal or better efficacy and an acceptable toxicity profile. We aimed to confirm the non-inferiority of GS to GC for patients with advanced/recurrent BTC in terms of overall survival (OS). We undertook a phase III randomized trial in 33 institutions in Japan. Between May 2013 and March 2016, 354 patients were enrolled. GS was found to be non-inferior to GC [median OS: 13.4 months with GC and 15.1 months with GS, HR, 0.945; 90% confidence interval (CI), 0.78-1.15; P = 0.046 for non-inferiority]. The median PFS was 5.8 months with GC and 6.8 months with GS (HR 0.86; 95% CI 0.70-1.07). The RR was 32.4% with GC and 29.8% with GS. Both treatments were generally well-tolerated. Clinically significant AEs were observed in 35.1% of patients in the GC arm and 29.9% in the GS arm. GS, which does not require hydration, should be considered a new, convenient standard of care option for patients with advanced/recurrent BTC.
We have investigated the prevalence and types of germline mutations in patients with biliary tract carcinoma (BTC), reviewed 269 patients with pathologically proven BTC, and collected clinical characteristics, including medical and family histories. Three pathogenic mutations in three patients were identified: two in BRCA2 and one in BRCA1. However, no mutation in mismatch-repair genes were detected, despite 63 patients meeting modified Revised Bethesda Guidelines screening criteria and 18 qualifying as young BTC patients. We detected a high prevalence of pathogenic germline mutations in BRCA1/2 and none in mismatch-repair genes in BTC patients following enrichment according to family or medical history in this study.
Clinical trials
33 clinical studies are ongoing, including 5 phase I studies, 15 phase II studies, 6 phase III studies (such as adjuvant chemotherapy after resection versus resection alone for patients with resectable tumors, and chemotherapy with a new regimen versus standard therapy for patients with advanced tumors), and several observation or registration trials. Our studies are supported by the National Cancer Center Research and Development Fund (2020-J-3), Project for Development of Innovative Research on Cancer Therapeutics 17824440, 17824973, 14524743) from the Japan Agency for Medical Research and Development.
Education
Our staff members are working closely with residents to support their skill development and knowledge expansion in both clinical and research fields. We are conducting conferences daily for clinical practice and weekly for research development. The residents in our department published 5 papers as a first author in peer-reviewed journals in 2020, and are performing 6 planning or ongoing studies as a leading researcher, with assistance from staff members.
Future Prospects
Our department keeps providing the best and latest diagnosis, treatment and supportive care, and developing more effective methods and techniques for all patients with hepatobiliary and pancreatic cancer in this country and around the world. Among our activities, conducting clinical trials with novel promising agents for this disease is considered one of the most important tasks, and establishment of cutting-edge endoscopic procedures in this field is our most significant mission.
List of papers published in 2020
Journal
1. Bun S, Yonemori K, Sunadoi H, Nishigaki R, Noguchi E, Okusaka T, Nishida T, Fujiwara Y. Safety and Evidence of Off-Label Use of Approved Drugs at the National Cancer Center Hospital in Japan. JCO Oncol Pract, 17:e416-e425, 2021
2. Shibayama T, Makise N, Motoi T, Mori T, Hiraoka N, Yonemori K, Watanabe SI, Esaki M, Morizane C, Okuma T, Kawai A, Ushiku T, Yatabe Y, Yoshida A. Clinicopathologic Characterization of Epithelioid Hemangioendothelioma in a Series of 62 Cases: A Proposal of Risk Stratification and Identification of a Synaptophysin-positive Aggressive Subset. Am J Surg Pathol, 45:616-626, 2021
3. Fukunaga K, Hishinuma E, Hiratsuka M, Kato K, Okusaka T, Saito T, Ikeda M, Yoshida T, Zembutsu H, Iwata N, Mushiroda T. Determination of novel CYP2D6 haplotype using the targeted sequencing followed by the long-read sequencing and the functional characterization in the Japanese population. J Hum Genet, 66:139-149, 2021
4. Hijioka S, Okusaka T. Enormous Potential of Endoscopic Ultrasound-guided Liver Biopsies. Intern Med, 60:1655-1656, 2021
5. Koga T, Hijioka S, Ishikawa Y, Ito K, Harai S, Okusaka T, Saito Y. Duckbill-type antireflux self-expandable metal stent placement for post-choledochojejunostomy reflux cholangitis. Endoscopy, 53:E174-E176, 2021
6. Koga T, Hijioka S, Hisada Y, Maruki Y, Nagashio Y, Okusaka T, Saito Y. Endoscopic ultrasound-guided choledochoduodenostomy without fistula dilation using a novel fully covered metallic stent with a 5.9-Fr ultra-thin delivery system. Endoscopy, 53:E223-E225, 2021
7. Maruki Y, Morizane C, Arai Y, Ikeda M, Ueno M, Ioka T, Naganuma A, Furukawa M, Mizuno N, Uwagawa T, Takahara N, Kanai M, Asagi A, Shimizu S, Miyamoto A, Yukisawa S, Kadokura M, Kojima Y, Furuse J, Nakajima TE, Sudo K, Kobayashi N, Hama N, Yamanaka T, Shibata T, Okusaka T. Molecular detection and clinicopathological characteristics of advanced/recurrent biliary tract carcinomas harboring the FGFR2 rearrangements: a prospective observational study (PRELUDE Study). J Gastroenterol, 56:250-260, 2021
8. Maruki Y, Morizane C, Arai Y, Ikeda M, Ueno M, Ioka T, Naganuma A, Furukawa M, Mizuno N, Uwagawa T, Takahara N, Kanai M, Asagi A, Shimizu S, Miyamoto A, Yukisawa S, Kadokura M, Kojima Y, Furuse J, Nakajima TE, Sudo K, Kobayashi N, Hama N, Yamanaka T, Shibata T, Okusaka T. Correction to: Molecular detection and clinicopathological characteristics of advanced/recurrent biliary tract carcinomas harboring the FGFR2 rearrangements: a prospective observational study (PRELUDE Study). J Gastroenterol, 56:297, 2021
9. Maehara K, Hijioka S, Saito Y. Endoscopic ultrasound-guided hepatico-gastrictubestomy for bile duct stent obstruction in a patient with recurrent cancer after esophageal cancer surgery with gastric tube reconstruction. Dig Endosc, 33:466-467, 2021
10. Kitamura H, Hijioka S, Maruki Y, Ohba A, Nagashio Y, Okusaka T, Saito Y. Novel double endoscopic ultrasound-guided hepaticogastrostomy for two-hole benign anastomotic stenosis with difficult gastrointestinal approach. Endoscopy, 53:E140-E142, 2021
11. Yamamoto M, Yoshida M, Furuse J, Sano K, Ohtsuka M, Yamashita S, Beppu T, Iwashita Y, Wada K, Nakajima TE, Sakamoto K, Hayano K, Mori Y, Asai K, Matsuyama R, Hirashita T, Hibi T, Sakai N, Tabata T, Kawakami H, Takeda H, Mizukami T, Ozaka M, Ueno M, Naito Y, Okano N, Ueno T, Hijioka S, Shikata S, Ukai T, Strasberg S, Sarr MG, Jagannath P, Hwang TL, Han HS, Yoon YS, Wang HJ, Luo SC, Adam R, Gimenez M, Scatton O, Oh DY, Takada T. Clinical practice guidelines for the management of liver metastases from extrahepatic primary cancers 2021. J Hepatobiliary Pancreat Sci, 28:1-25, 2021
12. Ueno M, Morinaga S, Hashimoto Y, Umemoto K, Sasahira N, Saiura A, Seyama Y, Honda G, Ioka T, Takahashi H, Miyamoto A, Nakamori S, Unno M, Takadate T, Mizuno N, Shimizu Y, Ueno H, Sugiyama M, Fukutomi A, Shimizu S, Okusaka T, Furuse J. Tolerability of Nab-Paclitaxel Plus Gemcitabine as Adjuvant Setting in Japanese Patients With Resected Pancreatic Cancer: Phase I Study. Pancreas, 50:83-88, 2021
13. Ueno M, Morizane C, Furukawa M, Sakai D, Komatsu Y, Nakai Y, Tsuda M, Ozaka M, Mizuno N, Muto M, Fukutomi A, Ikeda M, Tsuji A, Katanuma A, Moriwaki T, Kajiwara T, Ishii H, Negoro Y, Shimizu S, Nemoto N, Kobayashi S, Makino K, Furuse J. A randomized, double-blind, phase II study of oral histone deacetylase inhibitor resminostat plus S-1 versus placebo plus S-1 in biliary tract cancers previously treated with gemcitabine plus platinum-based chemotherapy. Cancer Med, 10:2088-2099, 2021
14. Ioka T, Furuse J, Fukutomi A, Mizusawa J, Nakamura S, Hiraoka N, Ito Y, Katayama H, Ueno M, Ikeda M, Sugimori K, Okano N, Shimizu K, Yanagimoto H, Okusaka T, Ozaka M, Todaka A, Nakamori S, Tobimatsu K, Sata N, Kawashima Y, Hosokawa A, Yamaguchi T, Miyakawa H, Hara H, Mizuno N, Ishii H. Randomized phase II study of chemoradiotherapy with versus without induction chemotherapy for locally advanced pancreatic cancer: Japan Clinical Oncology Group trial, JCOG1106. Jpn J Clin Oncol, 51:235-243, 2021
15. Ikeda M, Okusaka T, Ohno I, Mitsunaga S, Kondo S, Ueno H, Morizane C, Gemmoto K, Suna H, Ushida Y, Furuse J. Phase I studies of peptide vaccine cocktails derived from GPC3, WDRPUH and NEIL3 for advanced hepatocellular carcinoma. Immunotherapy, 13:371-385, 2021
16. Nagino M, Hirano S, Yoshitomi H, Aoki T, Uesaka K, Unno M, Ebata T, Konishi M, Sano K, Shimada K, Shimizu H, Higuchi R, Wakai T, Isayama H, Okusaka T, Tsuyuguchi T, Hirooka Y, Furuse J, Maguchi H, Suzuki K, Yamazaki H, Kijima H, Yanagisawa A, Yoshida M, Yokoyama Y, Mizuno T, Endo I. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J Hepatobiliary Pancreat Sci, 28:26-54, 2021
17. Okano N, Furuse J, Ueno M, Morizane C, Yamanaka T, Ojima H, Ozaka M, Sasaki M, Takahara N, Nakai Y, Kobayashi S, Morimoto M, Hosoi H, Maeno S, Nagashima F, Ikeda M, Okusaka T. Multicenter Phase II Trial of Axitinib Monotherapy for Gemcitabine-Based Chemotherapy Refractory Advanced Biliary Tract Cancer (AX-BC Study). Oncologist, 26:97-e201, 2021
18. Takahashi H, Ikeda M, Shiba S, Imaoka H, Todaka A, Shioji K, Yane K, Kojima Y, Kobayashi S, Asagi A, Ozaka M, Takada R, Nagashio Y, Horiguchi S, Kasuga A, Suzuki E, Terashima T, Ueno M, Morizane C, Furuse J. Multicenter Retrospective Analysis of Chemotherapy for Advanced Pancreatic Acinar Cell Carcinoma: Potential Efficacy of Platinum- and Irinotecan-Containing Regimens. Pancreas, 50:77-82, 2021
19. Umemoto K, Takahashi H, Morizane C, Yamada I, Shimizu S, Shioji K, Yoshida Y, Motoya M, Mizuno N, Kojima Y, Terashima T, Uesugi K, Ueno M, Furuse J, Akimoto T, Ikeda M. FOLFIRINOX in advanced pancreatic cancer patients with the double-variant type of UGT1A1 *28 and *6 polymorphism: a multicenter, retrospective study. Cancer Chemother Pharmacol, 87:397-404, 2021
20. Imaoka H, Ikeda M, Maehara K, Umemoto K, Ozaka M, Kobayashi S, Terashima T, Inoue H, Sakaguchi C, Tsuji K, Shioji K, Okamura K, Tsujimoto A, Nakamura I, Shirakawa H, Furukawa M, Ueno M, Morizane C, Furuse J. Risk stratification and prognostic factors in patients with unresectable undifferentiated carcinoma of the pancreas. Pancreatology, 21:738-745, 2021
21. Aoki T, Kubota K, Kiritani S, Arita J, Morizane C, Masui T, Kudo A, Komoto I, Hatano E, Ito T, Osamura RY, Unno M, Uemoto S, Kokudo N. Survey of surgical resections for neuroendocrine liver metastases: A project study of the Japan Neuroendocrine Tumor Society (JNETS). J Hepatobiliary Pancreat Sci, 28:489-497, 2021
22. Yao JC, Strosberg J, Fazio N, Pavel ME, Bergsland E, Ruszniewski P, Halperin DM, Li D, Tafuto S, Raj N, Campana D, Hijioka S, Raderer M, Guimbaud R, Gajate P, Pusceddu S, Reising A, Degtyarev E, Shilkrut M, Eddy S, Singh S. Spartalizumab in metastatic, well/poorly-differentiated neuroendocrine neoplasms. Endocr Relat Cancer, 2021
23. Mukai Y, Ueno H. Establishment and implementation of Cancer Genomic Medicine in Japan. Cancer Sci, 112:970-977, 2021
24. Shimoyama R, Hijioka S, Mizuno N, Ogawa G, Kataoka T, Katayama H, Machida N, Honma Y, Boku N, Hamaguchi T, Fukuda H, Terashima M, Kanemitsu Y, Furuse J. Study protocol for a multi-institutional randomized phase III study comparing combined everolimus plus lanreotide therapy and everolimus monotherapy in patients with unresectable or recurrent gastroenteropancreatic neuroendocrine tumors; Japan Clinical Oncology Group Study JCOG1901 (STARTER-NET study). Pancreatology, 20:1183-1188, 2020
25. Ito T, Fujimori N, Honma Y, Kudo A, Hijioka S, Katsushima S, Kimura Y, Fukutomi A, Hisamatsu S, Nakajima A, Shimatsu A. Long-term safety and efficacy of lanreotide autogel in Japanese patients with neuroendocrine tumors: Final results of a phase II open-label extension study. Asia Pac J Clin Oncol, 2020
26. Nakamura Y, Taniguchi H, Ikeda M, Bando H, Kato K, Morizane C, Esaki T, Komatsu Y, Kawamoto Y, Takahashi N, Ueno M, Kagawa Y, Nishina T, Kato T, Yamamoto Y, Furuse J, Denda T, Kawakami H, Oki E, Nakajima T, Nishida N, Yamaguchi K, Yasui H, Goto M, Matsuhashi N, Ohtsubo K, Yamazaki K, Tsuji A, Okamoto W, Tsuchihara K, Yamanaka T, Miki I, Sakamoto Y, Ichiki H, Hata M, Yamashita R, Ohtsu A, Odegaard JI, Yoshino T. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med, 26:1859-1864, 2020
27. Zenda S, Ryu A, Takashima A, Arai M, Takagi Y, Miyaji T, Mashiko T, Shimizu Y, Yamazaki N, Morizane C, Yamaguchi T, Kawaguchi T, Hanai A, Uchitomi Y, Oshiba F. Hydrocolloid dressing as a prophylactic use for hand-foot skin reaction induced by multitargeted kinase inhibitors: protocol of a phase 3 randomised self-controlled study. BMJ Open, 10:e038276, 2020
28. Nagashio Y, Hijioka S, Kanai Y, Ohba A, Maruki Y, Okusaka T, Saito Y. Novel side-by-side metal stent placement for recurrent hepatic hilar obstruction after placement of multiple metal stents. Endoscopy, 52:E330-E332, 2020
29. Maehara K, Hijioka S, Nagashio Y, Ohba A, Kanai Y, Okusaka T, Saito Y. Simultaneous endoscopic ultrasound-guided hepaticogastrostomy and bridging stenting with partial stent-in-stent method. Endoscopy, 52:E381-E382, 2020
30. Lin Y, Nakatochi M, Hosono Y, Ito H, Kamatani Y, Inoko A, Sakamoto H, Kinoshita F, Kobayashi Y, Ishii H, Ozaka M, Sasaki T, Matsuyama M, Sasahira N, Morimoto M, Kobayashi S, Fukushima T, Ueno M, Ohkawa S, Egawa N, Kuruma S, Mori M, Nakao H, Adachi Y, Okuda M, Osaki T, Kamiya S, Wang C, Hara K, Shimizu Y, Miyamoto T, Hayashi Y, Ebi H, Kohmoto T, Imoto I, Kasugai Y, Murakami Y, Akiyama M, Ishigaki K, Matsuda K, Hirata M, Shimada K, Okusaka T, Kawaguchi T, Takahashi M, Watanabe Y, Kuriki K, Kadota A, Okada R, Mikami H, Takezaki T, Suzuki S, Yamaji T, Iwasaki M, Sawada N, Goto A, Kinoshita K, Fuse N, Katsuoka F, Shimizu A, Nishizuka SS, Tanno K, Suzuki K, Okada Y, Horikoshi M, Yamauchi T, Kadowaki T, Yu H, Zhong J, Amundadottir LT, Doki Y, Ishii H, Eguchi H, Bogumil D, Haiman CA, Le Marchand L, Mori M, Risch H, Setiawan VW, Tsugane S, Wakai K, Yoshida T, Matsuda F, Kubo M, Kikuchi S, Matsuo K. Genome-wide association meta-analysis identifies GP2 gene risk variants for pancreatic cancer. Nat Commun, 11:3175, 2020
31. Nara S, Esaki M, Ban D, Takamoto T, Shimada K, Ioka T, Okusaka T, Ishii H, Furuse J. Adjuvant and neoadjuvant therapy for biliary tract cancer: a review of clinical trials. Jpn J Clin Oncol, 50:1353-1363, 2020
32. Okusaka T, Furuse J. Recent advances in chemotherapy for pancreatic cancer: evidence from Japan and recommendations in guidelines. J Gastroenterol, 55:369-382, 2020
33. Maruki Y, Hijioka S, Wu SYS, Ohba A, Nagashio Y, Kondo S, Morizane C, Ueno H, Okusaka T, Saito Y. Novel endoscopic technique for trisegment drainage in patients with unresectable hilar malignant biliary strictures (with video). Gastrointest Endosc, 92:763-769, 2020
34. Hisada Y, Hijioka S, Ohba A, Nagashio Y, Kanai Y, Okusaka T, Saito Y. Novel endoscopic ultrasound-guided hepaticoduodenostomy using a forward-viewing echoendoscope for altered anatomy. Endoscopy, 2020
35. Maehara K, Hijioka S, Nagashio Y, Ohba A, Maruki Y, Suzuki H, Sone M, Okusaka T, Saito Y. Endoscopic ultrasound-guided hepaticogastrostomy or hepaticojejunostomy without dilation using a stent with a thinner delivery system. Endosc Int Open, 8:E1034-E1038, 2020
36. Maehara K, Hijioka S, Sakamoto T, Maruki Y, Tamada K, Okusaka T, Saito Y. Novel biliary drainage of a choledochojejunal anastomotic stenosis using a double-balloon endoscope and forward-viewing endoscopic ultrasound. Endoscopy, 2020
37. Ohmoto A, Morizane C. Genomic Profiles and Current Therapeutic Agents in Neuroendocrine Neoplasms. Curr Drug Targets, 21:389-405, 2020
38. Ikeda M, Morizane C, Hijioka S, Matsumoto S, Konishi T, Komoto I, Aoki T, Ito T, Furuse J, Sasano H, Doi R. Optimal strategy of systemic treatment for unresectable pancreatic neuroendocrine tumors based upon opinion of Japanese experts. Pancreatology, 20:944-950, 2020
39. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut, 69:1492-1501, 2020
40. Kudo M, Okusaka T, Motomura K, Ohno I, Morimoto M, Seo S, Wada Y, Sato S, Yamashita T, Furukawa M, Aramaki T, Nadano S, Ohkawa K, Fujii H, Kudo T, Furuse J, Takai H, Homma G, Yoshikawa R, Zhu AX. Ramucirumab after prior sorafenib in patients with advanced hepatocellular carcinoma and elevated alpha-fetoprotein: Japanese subgroup analysis of the REACH-2 trial. J Gastroenterol, 55:627-639, 2020
41. Kudo M, Morimoto M, Moriguchi M, Izumi N, Takayama T, Yoshiji H, Hino K, Oikawa T, Chiba T, Motomura K, Kato J, Yasuchika K, Ido A, Sato T, Nakashima D, Ueshima K, Ikeda M, Okusaka T, Tamura K, Furuse J. A randomized, double-blind, placebo-controlled, phase 3 study of tivantinib in Japanese patients with MET-high hepatocellular carcinoma. Cancer Sci, 111:3759-3769, 2020
42. Kudo M, Galle PR, Llovet JM, Finn RS, Vogel A, Motomura K, Assenat E, Merle P, Brandi G, Daniele B, Okusaka T, Tom??ek J, Borg C, Dadduzio V, Morimoto M, Pracht M, Jen MH, Drove Ubreva N, Widau RC, Shinozaki K, Yoshikawa R, Zhu AX. Ramucirumab in elderly patients with hepatocellular carcinoma and elevated alpha-fetoprotein after sorafenib in REACH and REACH-2. Liver Int, 40:2008-2020, 2020
43. Athauda A, Fong C, Lau DK, Javle M, Abou-Alfa GK, Morizane C, Steward K, Chau I. Broadening the therapeutic horizon of advanced biliary tract cancer through molecular characterisation. Cancer Treat Rev, 86:101998, 2020
44. Sugawara S, Sone M, Morita S, Hijioka S, Sakamoto Y, Kusumoto M, Arai Y. Radiologic Assessment for Endoscopic US-guided Biliary Drainage. Radiographics, 40:667-683, 2020
45. Okano N, Morizane C, Nomura S, Takahashi H, Tsumura H, Satake H, Mizuno N, Tsuji K, Shioji K, Asagi A, Yasui K, Kitagawa S, Kashiwada T, Ishiguro A, Kanai M, Ueno M, Ogura T, Shimizu S, Tobimatsu K, Motoya M, Nakashima K, Ikeda M, Okusaka T, Furuse J. Phase II clinical trial of gemcitabine plus oxaliplatin in patients with metastatic pancreatic adenocarcinoma with a family history of pancreatic/breast/ovarian/prostate cancer or personal history of breast/ovarian/prostate cancer (FABRIC study). Int J Clin Oncol, 25:1835-1843, 2020
46. Ohashi Y, Ikeda M, Kunitoh H, Sasako M, Okusaka T, Mukai H, Fujiwara K, Nakamura M, Oba MS, Kimura T, Ibusuki K, Sakon M. Venous thromboembolism in cancer patients: report of baseline data from the multicentre, prospective Cancer-VTE Registry. Jpn J Clin Oncol, 50:1246-1253, 2020
47. Ohashi Y, Ikeda M, Kunitoh H, Sasako M, Okusaka T, Mukai H, Fujiwara K, Nakamura M, Oba MS, Kimura T, Ibusuki K, Sakon M. Corrigendum to: Venous thromboembolism in cancer patients: report of baseline data from the multicentre, prospective Cancer-VTE Registry. Jpn J Clin Oncol, 50:1346, 2020
48. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol, 38:2960-2970, 2020
49. McNamara MG, Lopes A, Wasan H, Malka D, Goldstein D, Shannon J, Okusaka T, Knox JJ, Wagner AD, Andr? T, Cunningham D, Moehler M, Jensen LH, Koeberle D, Bekaii-Saab T, Bridgewater J, Valle JW. Landmark survival analysis and impact of anatomic site of origin in prospective clinical trials of biliary tract cancer. J Hepatol, 73:1109-1117, 2020
50. Shimizu Y, Hijioka S, Hirono S, Kin T, Ohtsuka T, Kanno A, Koshita S, Hanada K, Kitano M, Inoue H, Itoi T, Ueki T, Matsuo K, Yanagisawa A, Yamaue H, Sugiyama M, Okazaki K. New Model for Predicting Malignancy in Patients With Intraductal Papillary Mucinous Neoplasm. Ann Surg, 272:155-162, 2020
51. Zhu AX, Nipp RD, Finn RS, Galle PR, Llovet JM, Blanc JF, Okusaka T, Chau I, Cella D, Girvan A, Gable J, Bowman L, Wang C, Hsu Y, Abada PB, Kudo M. Ramucirumab in the second-line for patients with hepatocellular carcinoma and elevated alpha-fetoprotein: patient-reported outcomes across two randomised clinical trials. ESMO Open, 5:2020
52. Hiraoka N, Nitta H, Ohba A, Yoshida H, Morizane C, Okusaka T, Nara S, Esaki M, Kishi Y, Shimada K. Details of human epidermal growth factor receptor 2 status in 454 cases of biliary tract cancer. Hum Pathol, 105:9-19, 2020
53. Tanaka H, Hijioka S, Hosoda W, Ueno M, Kobayashi N, Ikeda M, Ito T, Kodama Y, Morizane C, Notohara K, Taguchi H, Kitano M, Komoto I, Tsuji A, Hashigo S, Kanno A, Miyabe K, Takagi T, Ishii H, Kojima Y, Yoshitomi H, Yanagimoto H, Furuse J, Mizuno N. Pancreatic neuroendocrine carcinoma G3 may be heterogeneous and could be classified into two distinct groups. Pancreatology, 20:1421-1427, 2020
54. Yokose T, Kabe Y, Matsuda A, Kitago M, Matsuda S, Hirai M, Nakagawa T, Masugi Y, Hishiki T, Nakamura Y, Shinoda M, Yagi H, Abe Y, Oshima G, Hori S, Nakano Y, Honda K, Kashiro A, Morizane C, Nara S, Kikuchi S, Shibahara T, Itonaga M, Ono M, Minegishi N, Koshiba S, Yamamoto M, Kuno A, Handa H, Sakamoto M, Suematsu M, Kitagawa Y. O-Glycan-Altered Extracellular Vesicles: A Specific Serum Marker Elevated in Pancreatic Cancer. Cancers (Basel), 12:2020
55. Takai E, Nakamura H, Chiku S, Kubo E, Ohmoto A, Totoki Y, Shibata T, Higuchi R, Yamamoto M, Furuse J, Shimizu K, Takahashi H, Morizane C, Furukawa T, Yachida S. Whole-exome Sequencing Reveals New Potential Susceptibility Genes for Japanese Familial Pancreatic Cancer. Ann Surg, 2020
56. Fujimori M, Sato A, Jinno S, Okusaka T, Yamaguchi T, Ikeda M, Ueno M, Ozaka M, Takayama Y, Miyaji T, Majima Y, Uchitomi Y. Integrated communication support program for oncologists, caregivers and patients with rapidly progressing advanced cancer to promote patient-centered communication: J-SUPPORT 1904 study protocol for a randomised controlled trial. BMJ Open, 10:e036745, 2020
57. Ueno M, Nakamori S, Sugimori K, Kanai M, Ikeda M, Ozaka M, Furukawa M, Okusaka T, Kawabe K, Furuse J, Komatsu Y, Ishii H, Sato A, Shimizu S, Chugh P, Tang R, Ioka T. nal-IRI+5-FU/LV versus 5-FU/LV in post-gemcitabine metastatic pancreatic cancer: Randomized phase 2 trial in Japanese patients. Cancer Med, 9:9396-9408, 2020
58. Ueno M, Ikeda M, Sasaki T, Nagashima F, Mizuno N, Shimizu S, Ikezawa H, Hayata N, Nakajima R, Morizane C. Phase 2 study of lenvatinib monotherapy as second-line treatment in unresectable biliary tract cancer: primary analysis results. BMC Cancer, 20:1105, 2020
59. Imaoka H, Ikeda M, Maehara K, Umemoto K, Ozaka M, Kobayashi S, Terashima T, Inoue H, Sakaguchi C, Tsuji K, Shioji K, Okamura K, Kawamoto Y, Suzuki R, Shirakawa H, Nagano H, Ueno M, Morizane C, Furuse J. Clinical outcomes of chemotherapy in patients with undifferentiated carcinoma of the pancreas: a retrospective multicenter cohort study. BMC Cancer, 20:946, 2020
60. Watanabe H, Yamazaki Y, Fujishima F, Izumi K, Imamura M, Hijioka S, Toriyama K, Yatabe Y, Kudo A, Motoi F, Unno M, Sasano H. O(6)-methylguanine DNA methyltransferase and glucose transporter 2 in foregut and hindgut gastrointestinal neuroendocrine neoplasms. BMC Cancer, 20:1195, 2020
