Annual Report 2020
Department of Laboratory Medicine
Hiromichi Matsushita, Kuniko Sunami, Minoru Kojima, Takahiro Nishino, Kimihiko Kawamura, Motoi Miyakoshi, Chiaki Hayashi, Yoji Hashimoto, Yasuo Shibuki, Hiroki Kakishima, Tomohiro Nakatani, Koji Yamada, Rie Matsuo, Kazuya Tokita, Chiaki Ikeda, Shuji Ota, Arisa Hanai, Yusuke Okui, Sayaka Takeuchi, Miyuki Hasumi, Sachiko Kobayashi, Satoe Miyaki, Noriko Takahashi, Mizuho Fujima, Tomoe Ito, Kyoko Orihara, Kaori Ueki, Fumie Watanabe, Akino Kino, Takako Takada, Kyoko Osanai, Ruriko Machida, Asuka Matsunaga, Hiroshi Chigira, Go Sato, Sakiko Yoshimura, Yu Aruga, Saori Kobayashi, Kaori Yamaguchi, Saori Nakabayashi, Shingo Nakajima, Hideya Matsubayashi, Saeko Shirahama, Akiko Takayanagi, Mei Fukuhara, Kumi Nakatani, Moemi Kasane, Kazuhiro Yoshida, Kenta Takehara, Madoka Kondo, Kana Katsuragi, Aisa Mizoguchi, Nao Iwashita, Mayu Takeno, Sakura Ishida, Misato Tsubokura, Haruka Katagiri, Ayaka Ichikawa, Yuka Yasuno, Yuki Minakawa, Kanako Kanaizuka, Minami Sato, Kaho Matsui, Takashi Kubo, Mayuko Kitami, Shigeru Tamura, Megumi Masuda, Hiyori Yatsu, Yuri Tanaka, Kana Miyajima, Misaki Sato, Chika Tokutake, Yuki Nakai, Wakana Tanno, Dai Mikami, Nozomi, Shishido, Manami Ito, Juri Hiraiwa, Nao Sato, Satono Shima, Aya Iwasaki, Yuma Sekiguchi, Ginga Nagasawa, Yoshiko Shibata, Ritsuko Toyama, Chieko Nozawa, Kazue Naoi, Kinue Tsubokawa, Kozaburo Endo, Ayumi Yokota
Introduction
The operations performed at the Department of Laboratory Medicine include clinical laboratory testing (urinalysis and other general testing, biochemistry, immunology, hematology, bacteriology, and gene testing), blood sampling, transfusion and cell therapy testing, physiological function examinations (ultrasonography, electrocardiogram, respiratory function test), and pathological examinations. The department has maintained its accreditation status under ISO 15189, the international standard defining the requirements for quality and competence in medical laboratories, since September 2012.
The number of clinical laboratory test orders decreased to 6,410,654 in 2020, corresponding to 95.0% of that in 2019, due to the outbreak of the COVID-19 virus (Table 1). However, it has been gradually recovering in recent days.
Routine activities
The Clinical Laboratory Testing Section has been providing the additional information based on the laboratory results, such as a corrected serum calcium concentration, ALBI score/grade, and the total number of eosinophils and basophils. In hematology, many tests have been performed to detect minimal residual disease in various hematopoietic malignancies using multi-color flow cytometers. In bacteriology, the identification of the species of microorganisms and the detection of CD toxins and drug resistance genes have been performed using gene analyzers to support the activity of the Infection Control Team. Particularly, the genetic test to detect the COVID-19 virus has been introduced and refined in cooperation with the gene testing team. In regard to gene testing, we have been holding expert panel meetings for cancer genomic medicine as a core hospital, based on the results of cancer gene panel sequencing; the number of examined cases has been increasing (Table 2). In addition, we have introduced Oncomine Dx Target Test NGS system to establish a system for rapid implementation of companion diagnostics. In blood sampling, we have been making an effort to shorten patient waiting time, aiming for less than 20 minutes in 85% of patients.
Table 1. Number of sessions and cancer genomic profiling tests in the expert panel meeting (2020)

Table 2. Number of clinical tests performed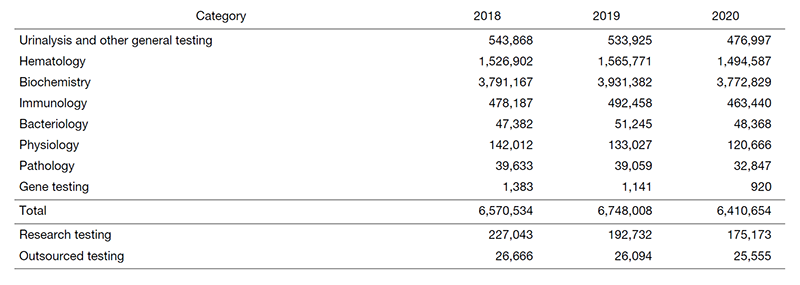

The Blood Transfusion Testing and Cell Therapy Section has automated the evaluation of blood type in shift duty and the process of ascites in cell-free and concentrated ascites reinfusion therapy (CART), and has managed the cell processing in chimeric antigen receptor T-cell (CAR-T) therapy, which has been gradually increasing.
The Physiological Examination Section has positively performed the evaluation of cardiotoxicity of anticancer agents and Sinusoidal Obstruction Syndrome/ Veno-Occlusive Disease (SOS/VOD) after hematopoietic stem cell transplantation.
The Pathological Examination Section has introduced the liquid-based cytology (LBC) in gynecological diseases for the efficient processing and the preparation for genetic tests.
Research activities
The Department of Laboratory Medicine is conducting basic and clinical studies concerning factors affecting the accuracy of laboratory tests, and has reported novel findings gained from the daily work. In biochemistry and immunology, we have been studying the stability and clinical use of specific tumor marker tests in collaboration with related department and manufacturers. We also contributed to our institutional observational study related to the COVID-19 infection. Our activity related to cancer genome profiling tests contributes to clinical studies in National Cancer Center Hospital and the cooperating hospitals, as well as to the decisions regarding cancer genomic medicine in Japan. The Blood Transfusion Testing and Cell Therapy Section has reported our experience of processing in CART, and contributed to clinical studies on CAR-T therapy.
Education
Medical technologists are required to provide the precise results of laboratory tests and examinations in each area of laboratory medicine. Human resource development is therefore conducted according to the education and training protocol prescribed in ISO 15189. Furthermore, the Department of Laboratory Medicine aims to cultivate the technique and knowledge of the medical technologists by positively supporting their qualifying examination and academic activities, although we have not been able to do so in 2020. We have received visits from many students who wish to become medical technologists and have encouraged their interest in laboratory medicine. In addition, a resident who aims to specialize in laboratory medicine has joined us this year.
Future Prospects
As an ISO 15189-accredited testing facility, we guarantee the quality and our ability to meet international standards and promote cooperation in clinical trials and clinical studies. In the near future, we will modify the system of the Clinical Laboratory Testing Section for efficient performance of laboratory tests using automated analyzers. Additionally, we will increase the personnel who specialize in cell therapy and flow cytometry analyses through professional education, in order to expand a capacity of these operations. Although the number of cancer genome profiling tests is expected to increase due to the insurance coverage of liquid biopsy in Japan, we will continue to manage the expert panel meetings as in previous years. Through these tasks, we will establish a clinical laboratory that can support each clinical department in communicating valuable information, while providing such information by ourselves.
List of papers published in 2020
Journal
1. Arakawa A, Ichikawa H, Kubo T, Motoi N, Kumamoto T, Nakajima M, Yonemori K, Noguchi E, Sunami K, Shiraishi K, Kakishima H, Yoshida H, Hishiki T, Kawakubo N, Kuroda T, Kiyokawa T, Yamada K, Yanaihara N, Takahashi K, Okamoto A, Hirabayashi S, Hasegawa D, Manabe A, Ono K, Matsuoka M, Arai Y, Togashi Y, Shibata T, Nishikawa H, Aoki K, Yamamoto N, Kohno T, Ogawa C. Vaginal Transmission of Cancer from Mothers with Cervical Cancer to Infants. N Engl J Med, 384:42-50, 2021
2. Sunami K, Naito Y, Aimono E, Amano T, Ennishi D, Kage H, Kanai M, Komine K, Koyama T, Maeda T, Morita S, Sakai D, Kohsaka S, Tsuchihara K, Yoshino T. The initial assessment of expert panel performance in core hospitals for cancer genomic medicine in Japan. Int J Clin Oncol, 26:443-449, 2021
3. Noda K, Matsuda K, Yagishita S, Maeda K, Akiyama Y, Terada-Hirashima J, Matsushita H, Iwata S, Yamashita K, Atarashi Y, Watanabe S, Ide N, Yoshida T, Ohmagari N, Mitsuya H, Hamada A. A novel highly quantitative and reproducible assay for the detection of anti-SARS-CoV-2 IgG and IgM antibodies. Sci Rep, 11:5198, 2021
4. Naito Y, Aburatani H, Amano T, Baba E, Furukawa T, Hayashida T, Hiyama E, Ikeda S, Kanai M, Kato M, Kinoshita I, Kiyota N, Kohno T, Kohsaka S, Komine K, Matsumura I, Miura Y, Nakamura Y, Natsume A, Nishio K, Oda K, Oda N, Okita N, Oseto K, Sunami K, Takahashi H, Takeda M, Tashiro S, Toyooka S, Ueno H, Yachida S, Yoshino T, Tsuchihara K. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (edition 2.1). Int J Clin Oncol, 26:233-283, 2021
5. Kojima M, Namikawa K, Kase Y, Matsushita H. Black Ascites. QJM, 114:523-524, 2021
6. Nagata Y, Kato K, Miyamoto T, Hirano H, Shoji H, Iwasa S, Honma Y, Takashima A, Hamaguchi T, Matsushita H, Nagashima K, Saruta M, Boku N. Safety and efficacy of cell-free and concentrated ascites reinfusion therapy (CART) in gastrointestinal cancer patients with massive ascites treated with systemic chemotherapy. Support Care Cancer, 28:5861-5869, 2020
7. Saito Y, Makita S, Chinen S, Kito M, Fujino T, Ida H, Hosoba R, Tanaka T, Fukuhara S, Munakata W, Suzuki T, Maruyama D, Miyagi-Maeshima A, Matsushita H, Izutsu K. Acute megakaryoblastic leukaemia with t(1;22)(p13?3;q13?1)/RBM15-MKL1 in an adult patient following a non-mediastinal germ cell tumour. Br J Haematol, 190:e329-e332, 2020
8. Hosoba R, Makita S, Shiotsuka M, Kobayashi O, Nakano K, Muroya M, Okada N, Suzuki M, Ida H, Fukuhara S, Munakata W, Suzuki T, Maruyama D, Maeshima AM, Matsushita H, Yamamoto N, Ohe Y, Iwata S, Izutsu K. COVID-19 pneumonia in a patient with adult T-cell leukemia-lymphoma. J Clin Exp Hematop, 60:174-178, 2020
9. Nishino T, Aruga Y, Ikeda C, Maeshima AM, Maruyama D, Matsushita H. Mixed-phenotype acute leukaemia consisting of five heterogeneous leukaemic populations without the expression of CD34. eJHaem, 1:406-407, 2020
10. Yatabe Y, Sunami K, Goto K, Nishio K, Aragane N, Ikeda S, Inoue A, Kinoshita I, Kimura H, Sakamoto T, Satouchi M, Shimizu J, Tsuta K, Toyooka S, Nishino K, Hatanaka Y, Matsumoto S, Mikubo M, Yokose T, Dosaka-Akita H. Multiplex gene-panel testing for lung cancer patients. Pathol Int, 70:921-931, 2020
