Annual Report 2020
Cancer Screening Center
Takahisa Matsuda, Masau Sekiguchi, Keiko Nakamura, Yasuo Kakugawa, Minori Matsumoto, Eriko Tsuruki, Masayoshi Yamada, Hiroyuki Takamaru, Takaaki Tsuchida, Masahiko Kusumoto, Gen Iinuma, Nachiko Uchiyama, Mari Kikuchi, Kimiteru Ito, Takahiro Morita, Hiroaki Kurihara, Miyuki Sone, Yasunori Mizuguchi, Hirokazu Watanabe, Mototaka Miyake, Syunsuke Sugawara, Yuko Kubo, Chihiro Ito, Nao Kikkawa, Shintaro Kimura, Sawako Kaku, Junji Omori, Mizuki Ozawa, Yuji Koretsune, Tomoyasu Kato, Mitsuya Ishikawa, Masaya Uno, Yasuhito Tanase, Mayumi Kato, Kenichi Nakamura, Hiroshi Katayama, Junko Eba, (Visiting Researcher) Ryutaro Kakinuma, Koichi Nagata, Shungo Endo, Kazutomo Togashi, Noriaki Takahashi, Takaaki Yasuda, Masaki Matsuoka, Ken Takabayashi, Hidenori Kanazawa, Shuji Yamamoto, Hidetsugu Yamagishi, Kayoko Kasuya, (Special research assistant) Mika Mori, Hiroko Shindo
Introduction
In the Cancer Screening Center (former name: Research Center for Cancer Prevention and Screening; RCCPS), we have provided broad opportunistic cancer screening using newly developed modalities since 2004. Most of the staff doctors hold two positions concurrently in both the Cancer Screening Center and their own specialized department. Our Cancer Screening Center consists of 20 radiologists, 8 gastroenterologists, 1 bronchoscopist, 5 gynecologists, 7 radiologic technologists, 5 ultrasound technologists and 4 nurses. Our department is in charge of multiphasic cancer screening using several imaging modalities to develop new cancer screening systems and to assess new screening tests.
The Team and What We Do
1. Cancer screening courses: Basic plan for males consists of screening for cancer of the lungs, esophagus, stomach, colorectum, liver, gall bladder, pancreas, kidneys, and prostate. The basic plan for females consists of the screening for cancer of the breast, uterus, and ovaries, in addition to the plan for males, excluding the prostate. In addition, PET (positron emission tomography) is provided as an option. In addition to multiphasic programs, the independent cancer screening program has been prepared for cancers of the lungs and female genitalia, including cancer of the uterus, ovaries, breast, and gastrointestinal tract. Blood samples are also obtained for biochemistry and tumor markers such as CA19-9, CEA, CA125, and PSA, as well as for genetic analysis.
2. Eligibility criteria for participants: Since 2013, the cancer screening program at the Cancer Screening Center has accepted applicants 40 years of age or older who give their written informed consent for the screening and blood samples for the genetic analysis and who take the survey regarding lifestyles. These study protocols have been approved by the Institutional Review Board (IRB). Applicants diagnosed with cancer and/or who have a history of cancer treatment, such as surgery, endoscopic mucosal resection, and chemotherapy within the previous one year, are excluded.
3. Cancer screening methods: On the first day, the multiphasic cancer screening programs (comprehensive cancer screening program) perform CT for lung cancer, abdominal US for cancer of the liver, gall bladder, pancreas, and kidneys, gynecological examinations with pap-smear and HPV test for uterine cancer, and MMG/Tomosynthesis and US for breast cancer. On the following day, gastroscopy for cancer of the esophagus and stomach, and total colonoscopy for cancer of the colon and rectum are conducted. Moreover, from the beginning of December 2010, CT-colonography (CTC) has been provided as an option for cancer screening. FDG-PET/CT is offered on the first day as an option, if the participants wish to undergo the examination. Furthermore, FDG-PET/MRI has been provided as an optional examination since 2018.
4. Number of cancer screening participants: The number of cancer screening participants between April 2020 and March 2021 is shown in this report (Tables 1 and 2). Due to the influence of COVID-19, the number of participants was remarkably decreased. A total of 1,597 people underwent cancer screening at the Cancer Screening Center during this period. Most of the participants (87%; n=1,391) chose the comprehensive cancer screening course. Regarding the data of cancer detection rate in each modality, we will report them in the near future.
Research activities
1. Study using gastric cancer screening data by upper gastrointestinal endoscopy
We retrospectively examined the data of 15,161 first-time examinees (male: 9,165, female: 5,996, mean age: 63 years) who underwent upper gastrointestinal endoscopy examination at the Cancer Screening Center, chronologically. Although there was no significant difference in gender or age during each target period, the prevalence of gastric cancer and atrophic gastritis clearly decreased over time (gastric cancer detection rate, 1.03%→0.69%→0.46%; atrophic gastritis positive rate, 72%→48%→37%).
Table 1: Number of participants of “Comprehensive cancer screening program”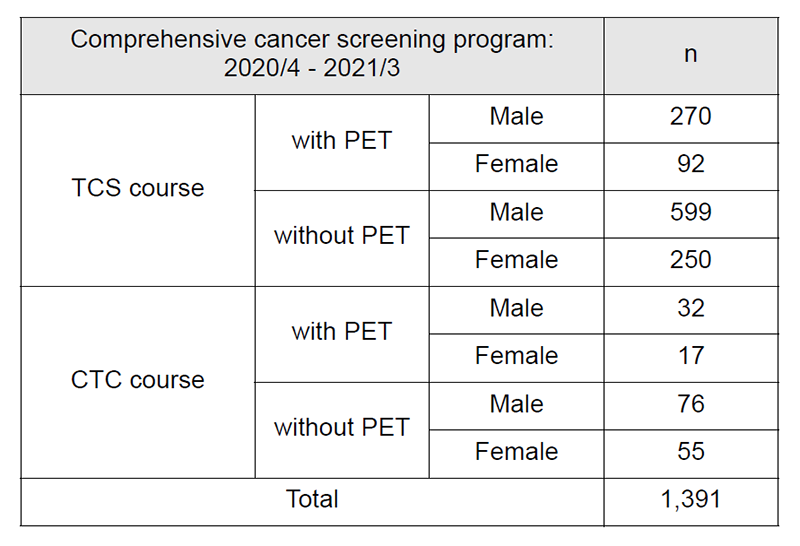

Table 2: Number of participants of the “Independent cancer screening course”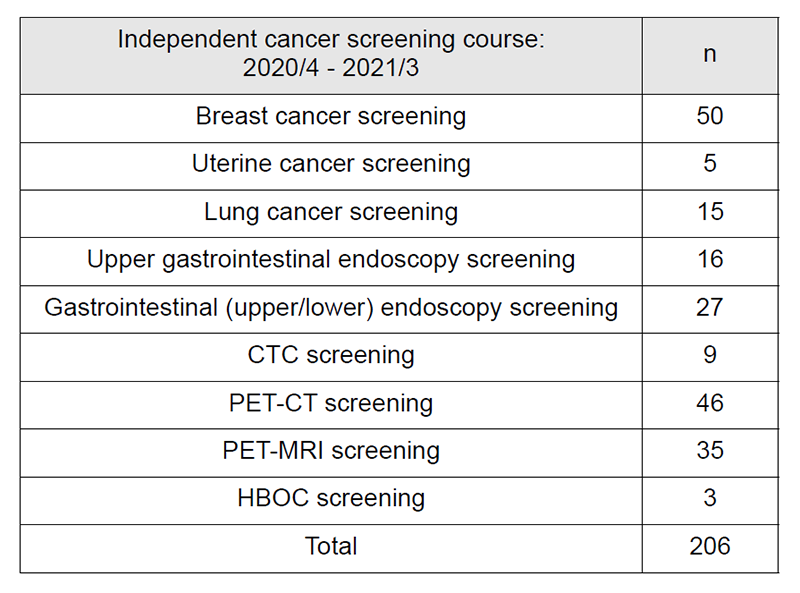

2. Study using colorectal cancer screening data by colonoscopy (CS)
(1) Data from screened individuals aged 40 to 54 years (n=2263) and 55 to 69 years (n=2621) who underwent their first-ever colonoscopy screening at the NCCH were analyzed. Among individuals aged 40 to 54 years, the prevalence of advanced colorectal neoplasia (ACN) was significantly higher in 249 individuals with affected first degree relatives (FDRs) than in those without (5.6% vs. 1.6%; P < .01). Among individuals aged 55 to 69 years, the prevalence of ACN was not significantly different between 291 individuals with affected FDRs and those without (5.8% vs. 5.8%; P Z .95). A family history of CRC in FDRs was associated with a higher prevalence of ACN in younger individuals. (Sekiguchi M, et al. Gastrointest Endosc 2020).
(2) The effectiveness in quality-adjusted life years, cost-effectiveness, and required number of CS procedures were evaluated for the screening strategies with primary CS screening, FIT, and the risk score, using a simulation model analysis. Screening strategies using the risk score can be more effective and cost-effective. (Sekiguchi M, et al. J Gastroenterol Hepatol 2020).
3. Accuracy of tumor markers (CEA, CA19-9) in cancer screening
We evaluated the diagnostic performance of serum carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 levels for (A) gastrointestinal and (B) multiple-organ cancer screening. The sensitivities of CEA and CA19-9 were low (CEA: 7.8% for A, 6.6% for B, CA19-9: 7.4% for A, 10.8% for B), thereby confirming their limited usefulness in multiple-organ cancer screening.
4. Amino-index (AICS) cancer screening accuracy evaluation study
Since the start of the research in July 2012, we have received plasma samples from 8,111 screening examinees and are proceeding with comprehensive amino acid analysis. In the near future, we will proceed with collation work with the examination data.
Clinical trials
We are conducting ongoing research based on the study protocol titled “Evaluation of effectiveness of cancer screening modality at the National Cancer Center”. The target modalities are as follows: 1) upper gastrointestinal endoscopy, 2) lower gastrointestinal endoscopy, 3) CT colonography, 4) chest computed tomography (CT), 5) sputum cytology, 6) mammography, 7) breast ultrasonography, 8) FDG-positron emission tomography (PET), 9) abdominal ultrasonography, and 10) serum tumor markers.
Future Prospects
Based on cancer screening data such as examination results, medical institution findings, follow-up findings, and the questionnaire survey concerning lifestyles for 10 years, we commenced to assess them with the support of the National Cancer Center Research and Development Fund.
