Annual Report 2020
Clinical Research Support Office
- Clinical Research Coordinating Division
Hideki Ueno, Miki Ito, Kikue Hasegawa, Asako Sakamoto, Emi Yasuda, Sho Murata, Mari Takahashi, Hiroko Kawaguchi, Shinobu Araki, Chiharu Nakano, Asako Kamikawa, Setsuko Kamizaki, Yoshimi Yamaguchi, Yumiko Ikuno, Kimiyo Yoshii, Hiroko Takagi, Maki Takayasu, Aya Matsumoto, Yukako Takasaki, Kayo Sawaki, Ran Obara, Shoko Kusaka, Harue Ui, Tamami Yamano, Kayo Ohsugi, Nahoko Hiraoka, Tomomi Tsuchiya, Chie Miyano, Hatsuki Ohno, Noriko Makita, Chikano Yashiro, Eri Yanagisawa, Yukari Nishiyama, Azumi Seko, Ai Sekido, Rie Nagao, Tomoko Morita, Mrie Komagata, Miki Shiraiwa, Aya Shiroichi, Anna Yashima, Hiroyuki Oyama, Ritsuko Yamamoto, Kazumi Higuchi, Ai Kobayakawa, Maya Niigawa, Hiroe Takayama, Mari Kondo, Tomoko Jinbo, Shiho Isobe, Junko Kojima, Chie Motegi, Kimiko Sega, Katsuaki Imaizumi, Nobuko Ushirozawa, Yoshiko Kanazu, Yoko Ebihara, Harumi Maruno, Junko Horie, Eiko Mimata, Kaori Matsushita, Yuriko Shinotsuka, Yki Sezaki, Satoko Murakami, Chiaki Yamamoto, Ken Kato, Teiko Yamane, Keiko Wakakuwa, Keiko Shimo, Mayumi Ikeda, Yuki Murai, Satomi Nakamori, Harumi Mochizuki, Riko Tamura, Sayaka Oba - Research Management Division
Kenichi Nakamura, Tomomi Hata, Makiko Watanabe, Mitsumi Terada, Takako Hitomi, Laureline Gatellier, Yoshiaki Kameda, Chie Igarashi, Kayo Motono, Natsuko Okita, Hisahiro Ito, Mamiko Kawasaki, Satoshi Kawashima, Hitomi Okuma, Naoko Matsui, Hitoshi Ozawa, Masahiko Ichimura, Miho Nakajima, Chiharu Mizoguchi, Mamiko Nakata, Hiroshi Katayama, Sawako Tomatsuri, Sachie Kawabata, Eiko Yorikane, Yoshie Shuda, Kenta Anjo, Kaori Izumino, Yuka Okamoto, Junko Eba, Keisuke Kanato, Tomoko Kataoka, Keita Sasaki, Hideki Masai, Kaori Umegai, Yuta Sekino, Taro Shibata, Aya Kuchiba, Junki Mizusawa, Ryunosuke Machida, Shun Sadachi, Akihiro Hirakawa, Seiji Sasaki, Kohei Uemura, Ryo Kitabayashi, Kanako Fuyama, Chihiro Yaita, Yuki Konda - Data Management Division
Haruhiko Fukuda, Harumi Kaba, Hiromi Katsuki, Yayoi Mitsumori, Tomoko Kojima, Mikio Mori, Nobuko Okamura, Ryuji Makiuchi, Keiko Ohata, Yukari Hoshina, Masahisa Kamikura, Yukari Nagasaka, Eiko Sayama, Eru Adachi, Minako Nagase, Kazumi Kurisita, Yuki Kazumi, Keiko Suto
Introduction
The Clinical Research Support Office supports clinical research conducted under the leadership by investigators in the National Cancer Center Hospital (NCCH). Supporting activities include protocol writing, central/local data management, statistical design and analysis, in-house/on-site monitoring, audits, patient recruitment, and other coordinating jobs.
The Team and What We Do
- Clinical Research Coordinating Division
The Clinical Research Coordinating Section and the Clinical Trial Administration Section support a lot of industry-sponsored registration trials as well as the physician-initiated registration-directed clinical trials. A total of 41 CRCs (clinical research coordinators), 9 CRC assistants, and 12 administration staff support these trials.
The Biobank and Translational Research Support Section has routinely obtained the informed consent to participate as an NCC biobank (NCCBB) donor from patients who consult with the NCCH for the first time. The CRCs in this section coordinate the translational research in several ways, such as assistance of registration for clinical trials, logistics of pathological specimens, data collection for case report forms, and coordination between sections. We explained the purport of the NCCBB to 7,420 patients from Apr 2020 to Mar 2021, and received consent for blood collection and use of their surplus samples for research from 6,713 patients (90.5% consent rate).
- Research Management Division
The Research Management Division is in charge of central research support functions: i) International clinical trial management, ii) Investigator-initiated registration-directed trial (Chiken) management, iii) Monitoring and consultation, iv) Multi-institutional clinical trial support, v) Biostatistics, vi) Pharmaceutical affairs consultation. The division has the capability to support various types of clinical trials covering both early phase ones including first-in-human trials and late phase multi-institutional trials. For the early phase trials, the division mainly offers comprehensive study management, site visit monitoring and safety information management. One of the strength of the division is that it can coordinate not only domestic trials but also international investigator-initiated registration-directed trials. The multi-institutional trial support function works as the Japan Clinical Oncology Group (JCOG) Operations Office which engages in protocol development, manuscript drafting, study coordination, etc., for late phase trials.
- Data Management Division
The Data Management Division is responsible for central data management and in-house study monitoring in investigator-initiated clinical trials for cancer therapeutic development. The Data Management Section supports early phase cancer trials mainly for drug development including registration trials which are led by physicians in the NCCH. The Multi-institutional Data Management Section supports mostly late development multi-modality multi-institutional phase II or phase III trials for adult solid cancer conducted by the JCOG.
Research activities
- Research Management Division
At academic conferences, several presentations were made by the section members on topics such as: i) NCCH initiatives as a Clinical Research Core Hospital; ii) Issues and solutions in facilitating clinical trials under the Clinical Trials Act; iii) Project management of investigator-initiated registration-directed trials, Advanced Medical Care studies, patient-proposed health services.
Clinical trials
- Clinical Research Coordinating Division
The number of industry-sponsored registration trials is increasing year by year, and the division supported 500 registration-directed clinical trials including 62 investigator-initiated registration-directed trials in FY2020 (Table 1).
- Research Management Division
The priority area of the division is genomic medicine, rare cancer and international trials. As of the end of the fiscal year 2020, the Research Management Division supported 20 investigator-initiated registration-directed trials, 11 clinical trials using Advanced Medical Care system and two trials under patient-proposed health services. This division has been in charge of the coordinating office of rare cancer registry study with basket sub-studies (MASTER KEY Project). This division also supports an international investigator-initiated registration-directed trial (PATHWAY trial).
Table 1. Trials Supported by Clinical Research Coordinating Division in FY2020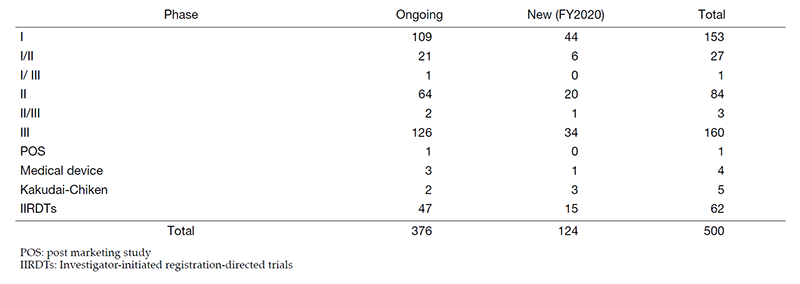

- Data Management Division
The Data Management Section supports 18 IND trials (8 open, 3 in preparation, 7 on follow-up) and 10 non-IND studies (4 open, 2 in preparation, 4 on follow-up). The Multi-institutional Data Management Section supports 101 non-IND trials (49 open, 15 in preparation, 37 on follow-up) as the JCOG Data Center.
Education
The staff members received not only day-to-day on-the-job training but also in-house educational seminars and the JCOG internal educational programs in order to learn clinical trial methodology, project management, monitoring, and research ethics.
Future Prospects
- Clinical Research Coordinating Division
The number of supported clinical trials is increasing as previously described, and the supporting area covered by CRCs will be expanded to include not only registration trials but also other investigator-initiated clinical trials. Therefore, the expansion of CRC staff members is highly demanded. In view of the plan for the NCCH, all members of this office will work together to contribute to reinforcing clinical research capabilities of the NCCH and to making this office a valuable unit for all members of our hospital.
The members of the Biobank and Translational Research Support Section received seminars related to clinical research ethics, etc. The section informs and educates investigators of the NCC about the NCC biobank and translational research periodically through the NCC University. As a future direction, the section will proceed with the improvement of quality of the NCC biobank's informatics and storage of serum. The section also aims to establish a support system for higher quality and quantity.
- Research Management Division
Since the number of investigator-initiated registration-directed trials has increased, reinforcement of staff resources is urgently needed. In response to this increase, this division will reinforce the support function for various clinical trials including international clinical trials, investigator-initiated registration-directed trials, Advanced Medical Care system trials, and patient-proposed health services trials. This division will also establish optimal ways to address the newly enacted Clinical Research Act.
- Data Management Division
The Data Management Division is introducing a web-based electronic data capturing (EDC) system and is promoting standardization of all aspects of data management, such as data format, case report forms and monitoring reports for increased data integrity, and cost effectiveness of daily work.
List of papers published in 2020
Journal
1. Hata T, Nakamura K, Yonemori K, Noguchi E, Watanabe M, Sohn J, Lu YS, Yap YS, Tamura K, Fujiwara Y. Regulatory and operational challenges in conducting Asian International Academic Trial for expanding the indications of cancer drugs. Clin Transl Sci, 14:1015-1025, 2021
2. Hattori A, Suzuki K, Takamochi K, Wakabayashi M, Aokage K, Saji H, Watanabe SI. Prognostic impact of a ground-glass opacity component in clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg, 161:1469-1480, 2021
3. Shimoyama R, Nakagawa K, Ishikura S, Wakabayashi M, Sasaki T, Yoshioka H, Hashimoto T, Kataoka T, Fukuda H, Watanabe SI. A multi-institutional randomized phase III trial comparing postoperative radiotherapy to observation after adjuvant chemotherapy in patients with pathological N2 Stage III non-small cell lung cancer: Japan Clinical Oncology Group Study JCOG1916 (J-PORT study). Jpn J Clin Oncol, 51:999-1003, 2021
4. Aokage K, Suzuki K, Wakabayashi M, Mizutani T, Hattori A, Fukuda H, Watanabe SI. Predicting pathological lymph node status in clinical stage IA peripheral lung adenocarcinoma. Eur J Cardiothorac Surg, 2021
5. Shimoyama R, Omori S, Nomura S, Kenmotsu H, Takahashi T, Harada H, Ishikura S, Mizutani T, Ando M, Kataoka T, Fukuda H, Ohe Y. A multi-institutional randomized phase III study comparing weekly carboplatin plus nab-paclitaxel and daily low-dose carboplatin as regimens for concurrent chemoradiotherapy in elderly patients with unresectable locally advanced non-small cell lung cancer: Japan Clinical Oncology Group Study JCOG1914. Jpn J Clin Oncol, 51:836-841, 2021
6. Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito S, Kaji M, Kimura Y, Hirao M, Yamada M, Kurita A, Takagi M, Lee SW, Takagane A, Yabusaki H, Hihara J, Boku N, Sano T, Sasako M. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer, 24:492-502, 2021
7. Tokunaga M, Kurokawa Y, Machida R, Sato Y, Takiguchi S, Doki Y, Yabusaki H, Watanabe M, Hato S, Nakamori M, Ito S, Yoshikawa T, Terashima M. Impact of postoperative complications on survival outcomes in patients with gastric cancer: exploratory analysis of a randomized controlled JCOG1001 trial. Gastric Cancer, 24:214-223, 2021
8. Fujitani K, Nakamura K, Mizusawa J, Kuwata T, Shimoda T, Katayama H, Kushima R, Taniguchi H, Yoshikawa T, Boku N, Terashima M, Fukuda H, Sano T, Sasako M. Posttherapy topographical nodal status, ypN-site, predicts survival of patients who received neoadjuvant chemotherapy followed by curative surgical resection for non-type 4 locally advanced gastric cancer: supplementary analysis of JCOG1004-A. Gastric Cancer, 24:197-204, 2021
9. Tanaka K, Ogawa G, Mizusawa J, Kadota T, Nakamura K, Shimada Y, Hamaguchi T, Fujita S, Kitano S, Inomata M, Kanemitsu Y, Fukuda H. Second primary cancers and recurrence in patients after resection of colorectal cancer: An integrated analysis of trials by Japan Clinical Oncology Group: JCOG1702A. Jpn J Clin Oncol, 51:185-191, 2021
10. Hara H, Mizusawa J, Hironaka S, Kato K, Daiko H, Abe T, Nakamura K, Ando N, Kitagawa Y. Influence of preoperative chemotherapy-induced leukopenia on survival in patients with esophageal squamous cell carcinoma: exploratory analysis of JCOG9907. Esophagus, 18:41-48, 2021
11. Takizawa K, Ono H, Hasuike N, Takashima A, Minashi K, Boku N, Kushima R, Katayama H, Ogawa G, Fukuda H, Fujisaki J, Oda I, Yano T, Hori S, Doyama H, Hirasawa K, Yamamoto Y, Ishihara R, Tanabe S, Niwa Y, Nakagawa M, Terashima M, Muto M. A nonrandomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010). Gastric Cancer, 24:479-491, 2021
12. Ioka T, Furuse J, Fukutomi A, Mizusawa J, Nakamura S, Hiraoka N, Ito Y, Katayama H, Ueno M, Ikeda M, Sugimori K, Okano N, Shimizu K, Yanagimoto H, Okusaka T, Ozaka M, Todaka A, Nakamori S, Tobimatsu K, Sata N, Kawashima Y, Hosokawa A, Yamaguchi T, Miyakawa H, Hara H, Mizuno N, Ishii H. Randomized phase II study of chemoradiotherapy with versus without induction chemotherapy for locally advanced pancreatic cancer: Japan Clinical Oncology Group trial, JCOG1106. Jpn J Clin Oncol, 51:235-243, 2021
13. Nishino K, Fujiwara Y, Ohe Y, Saito R, Miyauchi E, Kobayashi T, Nakai Y, Takahashi T, Shibata T, Hamaguchi T, Kikuchi K, Yamazaki N, Fukuda H, Nozawa K, Kiyohara Y. Results of the non-small cell lung cancer part of a phase III, open-label, randomized trial evaluating topical corticosteroid therapy for facial acneiform dermatitis induced by EGFR inhibitors: stepwise rank down from potent corticosteroid (FAEISS study, NCCH-1512). Support Care Cancer, 29:2327-2334, 2021
14. Maruyama D, Iida S, Ogawa G, Fukuhara N, Seo S, Miyazaki K, Yoshimitsu M, Kuroda J, Tsukamoto N, Tsujimura H, Hangaishi A, Yamauchi T, Utsumi T, Mizuno I, Takamatsu Y, Nagata Y, Minauchi K, Ohtsuka E, Hanamura I, Yoshida S, Yamasaki S, Suehiro Y, Kamiyama Y, Tsukasaki K, Nagai H. Randomised phase II study to optimise melphalan, prednisolone, and bortezomib in untreated multiple myeloma (JCOG1105). Br J Haematol, 192:531-541, 2021
15. Miyamoto K, Watanabe T, Wakabayashi M, Nakamura K, Watanabe Y, Maruyama D, Tobinai K, Tsukasaki K, Fukuda H. Comparison of the International Workshop Criteria and the Response Evaluation Criteria in Solid Tumors for indolent B-cell lymphoma. Int J Clin Oncol, 26:429-437, 2021
16. Kinoshita T, Watanabe T, Itoh K, Yoshimura K, Tobinai K, Ogura M, Yamaguchi M, Kurosawa M, Imaizumi Y, Ota S, Kaba H, Mukai K, Nakamura S, Ohshima K, Hotta T, Tsukasaki K, Nagai H, Shimoyama M. Clinical characteristics of patients with B-cell lymphoma enrolled in clinical trials for aggressive lymphoma in Japan: Japan Clinical Oncology Group - Lymphoma Study Group study - JCOG0108A. J Clin Exp Hematop, 61:35-41, 2021
17. Ohmachi K, Kinoshita T, Tobinai K, Ogawa G, Mizutani T, Yamauchi N, Fukuhara N, Uchida T, Yamamoto K, Miyazaki K, Tsukamoto N, Iida S, Utsumi T, Yoshida I, Imaizumi Y, Tokunaga T, Yoshida S, Masaki Y, Murayama T, Yakushijin Y, Suehiro Y, Nosaka K, Dobashi N, Kuroda J, Takamatsu Y, Maruyama D, Ando K, Ishizawa K, Ogura M, Yoshino T, Hotta T, Tsukasaki K, Nagai H. A randomized phase 2/3 study of R-CHOP vs CHOP combined with dose-dense rituximab for DLBCL: the JCOG0601 trial. Blood Adv, 5:984-993, 2021
18. Murakami N, Mori T, Machida R, Kodaira T, Ito Y, Shikama N, Konishi K, Matsumoto Y, Murakami Y, Nakamura N, Yamashita H, Yorozu A, Yoshimura M, Inoue K, Nozaki M, Ishikura S, Itami J, Nishimura Y, Kagami Y. Prognostic Value of Epithelial Cell Adhesion Molecules in T1-2N0M0 Glottic Cancer. Laryngoscope, 131:1522-1527, 2021
19. Bonomo P, Elad S, Kataoka T, Bossi P. The impact of the COVID-19 outbreak on supportive care for oral mucositis: current concepts and practice. Support Care Cancer, 29:2255-2258, 2021
20. Inokuchi J, Kuroiwa K, Nishiyama H, Kojima T, Kakehi Y, Sugimoto M, Takenaka A, Fujimoto K, Yamaguchi R, Habuchi T, Hashine K, Mizusawa J, Eba J, Naito S. Significance of the timing of ureteral ligation on prognosis during radical nephroureterectomy for upper urinary tract urothelial cancer. Int J Urol, 28:208-214, 2021
21. Iwatani T, Hara F, Shien T, Sasaki K, Katayama H, Fukuda H, Shiroiwa T, Iwata H. Prospective observational study estimating willingness-to-pay for breast cancer treatments through contingent valuation method in Japanese breast cancer patients (JCOG1709A). Jpn J Clin Oncol, 51:498-503, 2021
22. Kadota T, Minashi K, Wakabayashi M, Yano T, Ezoe Y, Tsuchida T, Ono H, Iizuka T, Matsuura N, Oda I, Takizawa K, Katayama H, Fukuda H, Muto M. Diagnostic yield of conventional endoscopy with endoscopic ultrasonography for submucosal invasion of superficial esophageal squamous cell carcinoma: a post hoc analysis of multicenter prospective confirmatory study (JCOG0508). Esophagus, 18:604-611, 2021
23. Kanemitsu Y, Shitara K, Mizusawa J, Hamaguchi T, Shida D, Komori K, Ikeda S, Ojima H, Ike H, Shiomi A, Watanabe J, Takii Y, Yamaguchi T, Katsumata K, Ito M, Okuda J, Hyakudomi R, Shimada Y, Katayama H, Fukuda H. Primary Tumor Resection Plus Chemotherapy Versus Chemotherapy Alone for Colorectal Cancer Patients With Asymptomatic, Synchronous Unresectable Metastases (JCOG1007; iPACS): A Randomized Clinical Trial. J Clin Oncol, 39:1098-1107, 2021
24. Katayama H, Mizusawa J, Fukuda H, Nakamura S, Nakamura K, Saijo N, Yokoyama A, Ohe Y, Shinkai T, Nakagawa K, Abe T, Mitsuoka S, Okamoto H, Yamamoto N, Yoshioka H, Ando M, Tamura T, Takeda K. Prognostic impact of geriatric assessment in elderly patients with non-small cell lung cancer: an integrated analysis of two randomized phase III trials (JCOG1115-A). Jpn J Clin Oncol, 51:685-692, 2021
25. Naito Y, Aburatani H, Amano T, Baba E, Furukawa T, Hayashida T, Hiyama E, Ikeda S, Kanai M, Kato M, Kinoshita I, Kiyota N, Kohno T, Kohsaka S, Komine K, Matsumura I, Miura Y, Nakamura Y, Natsume A, Nishio K, Oda K, Oda N, Okita N, Oseto K, Sunami K, Takahashi H, Takeda M, Tashiro S, Toyooka S, Ueno H, Yachida S, Yoshino T, Tsuchihara K. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (edition 2.1). Int J Clin Oncol, 26:233-283, 2021
26. Nozaki M, Kagami Y, Machida R, Nakamura K, Ito Y, Nishimura Y, Teshima T, Saito Y, Nagata Y, Matsumoto Y, Akimoto T, Hiraoka M. Final analysis of a Multicenter Single-Arm Confirmatory Trial of hypofractionated whole breast irradiation after breast-conserving surgery in Japan: JCOG0906. Jpn J Clin Oncol, 51:865-872, 2021
27. Natsume A, Aoki K, Ohka F, Maeda S, Hirano M, Adilijiang A, Motomura K, Sumi M, Nishikawa R, Narita Y, Muragaki Y, Maruyama T, Ito T, Beppu T, Nakamura H, Kayama T, Sato S, Nagane M, Mishima K, Nakasu Y, Kurisu K, Yamasaki F, Sugiyama K, Onishi T, Iwadate Y, Terasaki M, Kobayashi H, Matsumura A, Ishikawa E, Sasaki H, Mukasa A, Matsuo T, Hirano H, Kumabe T, Shinoura N, Hashimoto N, Aoki T, Asai A, Abe T, Yoshino A, Arakawa Y, Asano K, Yoshimoto K, Shibui S, Okuno Y, Wakabayashi T. Genetic analysis in patients with newly diagnosed glioblastomas treated with interferon-beta plus temozolomide in comparison with temozolomide alone. J Neurooncol, 148:17-27, 2020
28. Kenmotsu H, Niho S, Tsuboi M, Wakabayashi M, Ishii G, Nakagawa K, Daga H, Tanaka H, Saito H, Aokage K, Takahashi T, Menju T, Kasai T, Yoshino I, Minato K, Okada M, Eba J, Asamura H, Ohe Y, Watanabe SI. Randomized Phase III Study of Irinotecan Plus Cisplatin Versus Etoposide Plus Cisplatin for Completely Resected High-Grade Neuroendocrine Carcinoma of the Lung: JCOG1205/1206. J Clin Oncol, 38:4292-4301, 2020
29. Tanaka K, Tsutani Y, Wakabayashi M, Mizutani T, Aokage K, Miyata Y, Kuroda H, Saji H, Watanabe SI, Okada M. Sublobar resection versus lobectomy for patients with resectable stage I non-small cell lung cancer with idiopathic pulmonary fibrosis: a phase III study evaluating survival (JCOG1708, SURPRISE). Jpn J Clin Oncol, 50:1076-1079, 2020
30. Shimoyama R, Tsutani Y, Wakabayashi M, Katayama H, Fukuda H, Suzuki K, Watanabe SI. A multi-institutional randomized phase III trial comparing anatomical segmentectomy and wedge resection for clinical stage IA non-small cell lung cancer in high-risk operable patients: Japan Clinical Oncology Group Study JCOG1909 (ANSWER study). Jpn J Clin Oncol, 50:1209-1213, 2020
31. Nomura S, Goto Y, Mizutani T, Kataoka T, Kawai S, Okuma Y, Murakami H, Tanaka K, Ohe Y. A randomized phase III study comparing continuation and discontinuation of PD-1 pathway inhibitors for patients with advanced non-small-cell lung cancer (JCOG1701, SAVE study). Jpn J Clin Oncol, 50:821-825, 2020
32. Sato J, Satouchi M, Itoh S, Okuma Y, Niho S, Mizugaki H, Murakami H, Fujisaka Y, Kozuki T, Nakamura K, Nagasaka Y, Kawasaki M, Yamada T, Machida R, Kuchiba A, Ohe Y, Yamamoto N. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol, 21:843-850, 2020
33. Okamoto I, Nokihara H, Nomura S, Niho S, Sugawara S, Horinouchi H, Azuma K, Yoneshima Y, Murakami H, Hosomi Y, Atagi S, Ozaki T, Horiike A, Fujita Y, Okamoto H, Ando M, Yamamoto N, Ohe Y, Nakagawa K. Comparison of Carboplatin Plus Pemetrexed Followed by Maintenance Pemetrexed With Docetaxel Monotherapy in Elderly Patients With Advanced Nonsquamous Non-Small Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol, 6:e196828, 2020
34. Sato Y, Yamada T, Yoshikawa T, Machida R, Mizusawa J, Katayama H, Tokunaga M, Boku N, Terashima M. Randomized controlled Phase III trial to evaluate omentum preserving gastrectomy for patients with advanced gastric cancer (JCOG1711, ROAD-GC). Jpn J Clin Oncol, 50:1321-1324, 2020
35. Sato Y, Mizusawa J, Katayama H, Nakamura K, Fukagawa T, Katai H, Haruta S, Yamada M, Takagi M, Tamura S, Yoshimura T, Tokunaga M, Yoshikawa T, Boku N, Sano T, Sasako M, Terashima M. Diagnosis of invasion depth in resectable advanced gastric cancer for neoadjuvant chemotherapy: An exploratory analysis of Japan clinical oncology group study: JCOG1302A. Eur J Surg Oncol, 46:1074-1079, 2020
36. Tsukamoto S, Fujita S, Ota M, Mizusawa J, Shida D, Kanemitsu Y, Ito M, Shiomi A, Komori K, Ohue M, Akazai Y, Shiozawa M, Yamaguchi T, Bando H, Tsuchida A, Okamura S, Akagi Y, Takiguchi N, Saida Y, Akasu T, Moriya Y. Long-term follow-up of the randomized trial of mesorectal excision with or without lateral lymph node dissection in rectal cancer (JCOG0212). Br J Surg, 107:586-594, 2020
37. Kadota T, Ikematsu H, Sasaki T, Saito Y, Ito M, Mizutani T, Ogawa G, Shitara K, Ito Y, Kushima R, Kanemitsu Y, Muto M. Protocol for a single-arm confirmatory trial of adjuvant chemoradiation for patients with high-risk rectal submucosal invasive cancer after local resection: Japan Clinical Oncology Group Study JCOG1612 (RESCUE study). BMJ Open, 10:e034947, 2020
38. Kadota T, Tsukada Y, Ito M, Katayama H, Mizusawa J, Nakamura N, Ito Y, Bando H, Ando M, Onaya H, Fukuda H, Kanemitsu Y. A phase III randomized controlled trial comparing surgery plus adjuvant chemotherapy with preoperative chemoradiotherapy followed by surgery plus adjuvant chemotherapy for locally recurrent rectal cancer: Japan Clinical Oncology Group study JCOG1801 (RC-SURVIVE study). Jpn J Clin Oncol, 50:953-957, 2020
39. Miyamoto K, Wakabayashi M, Mizusawa J, Nakamura K, Katayama H, Higashi T, Inomata M, Kitano S, Fujita S, Kanemitsu Y, Fukuda H. Evaluation of the representativeness and generalizability of Japanese clinical trials for localized rectal/colon cancer: Comparing participants in the Japan Clinical Oncology Group study with patients in Japanese registries. Eur J Surg Oncol, 46:1642-1648, 2020
40. Shimoyama R, Hijioka S, Mizuno N, Ogawa G, Kataoka T, Katayama H, Machida N, Honma Y, Boku N, Hamaguchi T, Fukuda H, Terashima M, Kanemitsu Y, Furuse J. Study protocol for a multi-institutional randomized phase III study comparing combined everolimus plus lanreotide therapy and everolimus monotherapy in patients with unresectable or recurrent gastroenteropancreatic neuroendocrine tumors; Japan Clinical Oncology Group Study JCOG1901 (STARTER-NET study). Pancreatology, 20:1183-1188, 2020
41. Hironaka S, Komori A, Machida R, Ito Y, Takeuchi H, Ogawa G, Kato K, Onozawa M, Minashi K, Yano T, Nakamura K, Tsushima T, Hara H, Nozaki I, Ura T, Chin K, Fukuda H, Kitagawa Y. The association of primary tumor site with acute adverse event and efficacy of definitive chemoradiotherapy for cStage II/III esophageal cancer: an exploratory analysis of JCOG0909. Esophagus, 17:417-424, 2020
42. Nakajima TE, Yamaguchi K, Boku N, Hyodo I, Mizusawa J, Hara H, Nishina T, Sakamoto T, Shitara K, Shinozaki K, Katayama H, Nakamura S, Muro K, Terashima M. Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer, 23:677-688, 2020
43. Iwasa S, Okita N, Kuchiba A, Ogawa G, Kawasaki M, Nakamura K, Shoji H, Honma Y, Takashima A, Kato K, Hamaguchi T, Boku N, Yamada Y. Phase II study of lenvatinib for metastatic colorectal cancer refractory to standard chemotherapy: the LEMON study (NCCH1503). ESMO Open, 5:2020
44. Kagami Y, Yamamoto K, Shibata T, Tobinai K, Imaizumi Y, Uchida T, Shimada K, Minauchi K, Fukuhara N, Kobayashi H, Yamauchi N, Tsujimura H, Hangaishi A, Tominaga R, Suehiro Y, Yoshida S, Inoue Y, Suzuki S, Tokuhira M, Kusumoto S, Kuroda J, Yakushijin Y, Takamatsu Y, Kubota Y, Nosaka K, Morishima S, Nakamura S, Ogura M, Maruyama D, Hotta T, Morishima Y, Tsukasaki K, Nagai H. R-CHOP-14 versus R-CHOP-14/CHASER for upfront autologous transplantation in diffuse large B-cell lymphoma: JCOG0908 study. Cancer Sci, 111:3770-3779, 2020
45. Akagi T, Inomata M, Hara T, Mizusawa J, Katayama H, Shida D, Ohue M, Ito M, Kinugasa Y, Saida Y, Masaki T, Yamamoto S, Hanai T, Yamaguchi S, Watanabe M, Sugihara K, Fukuda H, Kanemitsu Y, Kitano S. Clinical impact of D3 lymph node dissection with left colic artery (LCA) preservation compared to D3 without LCA preservation: Exploratory subgroup analysis of data from JCOG0404. Ann Gastroenterol Surg, 4:163-169, 2020
46. Ito H, Suzuki K, Mizutani T, Aokage K, Wakabayashi M, Fukuda H, Watanabe SI. Long-term survival outcome after lobectomy in patients with clinical T1 N0 lung cancer. J Thorac Cardiovasc Surg, 2020
47. Katai H, Mizusawa J, Katayama H, Morita S, Yamada T, Bando E, Ito S, Takagi M, Takagane A, Teshima S, Koeda K, Nunobe S, Yoshikawa T, Terashima M, Sasako M. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol, 5:142-151, 2020
48. Kitamura H, Hinotsu S, Tsukamoto T, Shibata T, Mizusawa J, Kobayashi T, Miyake M, Nishiyama N, Kojima T, Nishiyama H. Effect of neoadjuvant chemotherapy on health-related quality of life in patients with muscle-invasive bladder cancer: results from JCOG0209, a randomized phase III study. Jpn J Clin Oncol, 50:1464-1469, 2020
49. Kunitoh H, Tsuboi M, Wakabayashi M, Okada M, Suzuki K, Watanabe S, Asamura H, Fukuda H, Shibata T, Kazato T, Mizutani T, Eba J. A phase III study of adjuvant chemotherapy in patients with completely resected, node-negative non-small cell lung cancer (JCOG 0707). JTCVS Open, 4:90-102, 2020
50. Moritani K, Kanemitsu Y, Shida D, Shitara K, Mizusawa J, Katayama H, Hamaguchi T, Shimada Y. A randomized controlled trial comparing primary tumour resection plus chemotherapy with chemotherapy alone in incurable stage IV colorectal cancer: JCOG1007 (iPACS study). Jpn J Clin Oncol, 50:89-93, 2020
51. Nishimura Y, Ishikura S, Shibata T, Kodaira T, Ito Y, Tsuchiya K, Murakami Y, Saitoh JI, Akimoto T, Nakata K, Yoshimura M, Teshima T, Toshiyasu T, Ota Y, Ishikawa K, Shimizu H, Minemura T, Nakamura K, Hiraoka M. A phase II study of adaptive two-step intensity-modulated radiation therapy (IMRT) with chemotherapy for loco-regionally advanced nasopharyngeal cancer (JCOG1015). Int J Clin Oncol, 25:1250-1259, 2020
52. Oashi K, Shibata T, Namikawa K, Takahashi A, Yokota K, Nakano E, Teramoto Y, Tsutsumida A, Maeda T, Yamazaki N. A single-arm confirmatory trial of pazopanib in patients with paclitaxel-pretreated primary cutaneous angiosarcoma: Japan Clinical Oncology Group study (JCOG1605, JCOG-PCAS protocol). BMC Cancer, 20:652, 2020
53. Onda T, Satoh T, Ogawa G, Saito T, Kasamatsu T, Nakanishi T, Mizutani T, Takehara K, Okamoto A, Ushijima K, Kobayashi H, Kawana K, Yokota H, Takano M, Kanao H, Watanabe Y, Yamamoto K, Yaegashi N, Kamura T, Yoshikawa H. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur J Cancer, 130:114-125, 2020
54. Sato Y, Kurokawa Y, Doki Y, Mizusawa J, Tanaka K, Katayama H, Boku N, Yoshikawa T, Terashima M. A Phase II study of preoperative chemotherapy with docetaxel, oxaliplatin and S-1 in gastric cancer with extensive lymph node metastasis (JCOG1704). Future Oncol, 16:31-38, 2020
55. Saunders DP, Rouleau T, Cheng K, Yarom N, Kandwal A, Joy J, Bektas Kayhan K, van de Wetering M, Brito-Dellan N, Kataoka T, Chiang K, Ranna V, Vaddi A, Epstein J, Lalla RV, Bossi P, Elad S. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer, 28:2473-2484, 2020
56. Suzuki K, Watanabe SI, Wakabayashi M, Saji H, Aokage K, Moriya Y, Yoshino I, Tsuboi M, Nakamura S, Nakamura K, Mitsudomi T, Asamura H. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg, 2020
57. Takahari D, Ito S, Mizusawa J, Katayama H, Terashima M, Sasako M, Morita S, Nomura T, Yamada M, Fujiwara Y, Kimura Y, Ikeda A, Kadokawa Y, Sano T. Long-term outcomes of preoperative docetaxel with cisplatin plus S-1 therapy for gastric cancer with extensive nodal metastasis (JCOG1002). Gastric Cancer, 23:293-299, 2020
58. Yano T, Hasuike N, Ono H, Boku N, Ogawa G, Kadota T, Oda I, Doyama H, Hori S, Iishi H, Takahashi A, Takizawa K, Muto M. Factors associated with technical difficulty of endoscopic submucosal dissection for early gastric cancer that met the expanded indication criteria: post hoc analysis of a multi-institutional prospective confirmatory trial (JCOG0607). Gastric Cancer, 23:168-174, 2020
59. Yokomizo A, Wakabayashi M, Satoh T, Hashine K, Inoue T, Fujimoto K, Egawa S, Habuchi T, Kawashima K, Ishizuka O, Shinohara N, Sugimoto M, Yoshino Y, Nihei K, Fukuda H, Tobisu KI, Kakehi Y, Naito S. Salvage Radiotherapy Versus Hormone Therapy for Prostate-specific Antigen Failure After Radical Prostatectomy: A Randomised, Multicentre, Open-label, Phase 3 Trial (JCOG0401)(†). Eur Urol, 77:689-698, 2020
