Annual Report 2020
Department of International Clinical Development
Kenichi Nakamura, Kan Yonemori, Tatsunori Shimoi, Seiichiro Abe, Hiroshi Igaki, Miyuki Sone, Shunsuke Tsukamoto, Hiroko Nakahama, Tomomi Hata, Yoichi Osato, Miho Nakajima, Kazuko Ohara, Hitomi Okuma, Yuta Maruki, Yusuke Okuma, Masamichi Takahashi, Koichi Ogura, Chiharu Mizoguchi, Kazuki Sudo, Shinji Kohsaka, Yuki Kojima, Sho Shiino, Hiroshi Yoshida, Keisuke Watanabe, Tomohiro Matsuda, Toshio Shimizu, Mitsumi Terada, Nobuko Ushirozawa, Hisahiro Itoh, Kumiko Yanagihara
Introduction
The Department of International Clinical Development (DICD) was established at the National Cancer Center Hospital to accelerate international research, education, and treatment under an integrated vision, and to promote the Asian Clinical Network Project (ATLAS project).
The Team and What We Do
(International Medical Care Section)
This section focuses on improving the quality of care and increasing the number of overseas patients at the NCCH by improving the infrastructure for the treatment of overseas patients. In FY2020, this section organized the intake of overseas patients, dealt with non-payment of medical fees, and improved the treatment efficiency in each department.
(International Professional Education Section)
In FY2020, as part of the ATLAS project, English content on genomic medicine, ISO15189 acquisition, phase I trials, and CRC education was developed on the ICRweb. In collaboration with PMDA, a Multi-regional Clinical Trials Seminar was held in January 2021 for participants from overseas regulatory authorities and overseas medical institutions.
(International Research and Development Section)
In FY2020, the MASTER KEY project was expanded to Asian countries, a platform trial for rare cancers. We also solicited applications for international clinical trials in Asia from investigators at NCCH and received 15 applications.
(International Translational Research Section)
In FY2021, the A-TRAIN study was developed, a TR study of liquid biopsy in which 10 Asian countries will participate. In addition, an observational study using MRD technique was planned in the field of breast cancer.
(Asian Partnerships Section)
As part of the ATLAS project, the NCC Asian Partnership Office is under development in Bangkok, Thailand, which will have a local coordination function for international clinical studies and will strengthen the network with local investigators, governments and regulatory authorities.
Research activities
Multiple presentations on the activity of the ATLAS project and DICD itself were made at the Global Health Conference, NAMOK-KFCR in Korea, DIA Global Oncology Development, the JSMO, etc.
Figure 1. Overview of the ATLAS project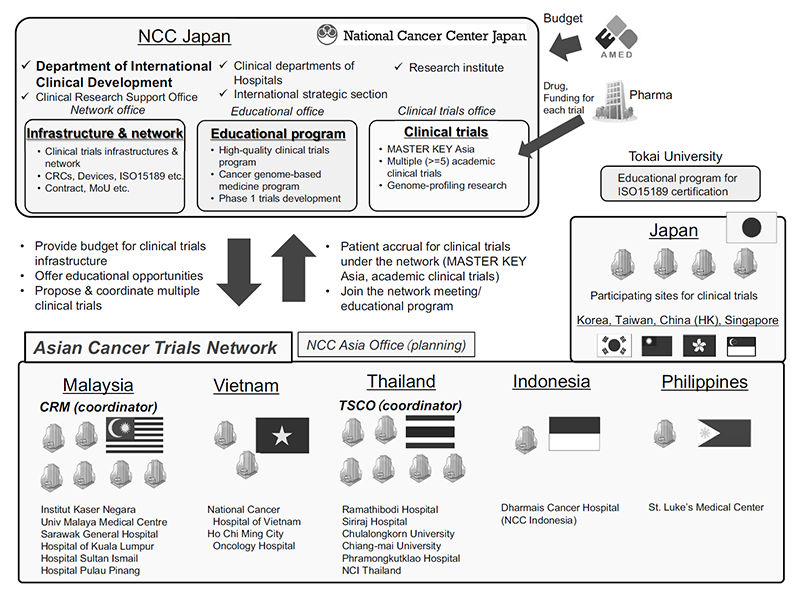

Clinical trials
Multiple international clinical trials are under preparation, which include MASTER KEY ASIA, a platform study for rare cancer development, the A-TRAIN study, a TR study of liquid biopsy with 10 Asian countries, and the TEAL trial, an investigator-initiated registration-directed trial for cervical cancer.
Education
Investigators and support staff were employed to strengthen the capability of international clinical trial development. Moreover, multiple educational contents in English are under preparation on the ICRweb.
Future Prospects
Our Mission
We collaborate with people around the world in the fields of research, education, and practice. We will expand our activities internationally to contribute to the improvement of the welfare of cancer patients.
Our Vision
1. To collaborate with Asian countries to conduct global clinical trials in a speedy, cost-effective manner.
2. To deploy advanced medical technologies, including cancer genomic treatment, to Asian and other countries.
3. To strengthen the international medical care function to provide excellent cancer treatment in Japan to patients around the world.
4. To form a strong network of researchers and research supporters through various educational programs.
5. To play a central role in the international deployment of Japan-originated medical technologies in cooperation with the government, regulatory authorities, pharmaceutical companies, and patient groups.
List of papers published in 2020
Journal
1. Hata T, Nakamura K, Yonemori K, Noguchi E, Watanabe M, Sohn J, Lu YS, Yap YS, Tamura K, Fujiwara Y. Regulatory and operational challenges in conducting Asian International Academic Trial for expanding the indications of cancer drugs. Clin Transl Sci, 14:1015-1025, 2021
2. Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito S, Kaji M, Kimura Y, Hirao M, Yamada M, Kurita A, Takagi M, Lee SW, Takagane A, Yabusaki H, Hihara J, Boku N, Sano T, Sasako M. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer, 24:492-502, 2021
3. Fujitani K, Nakamura K, Mizusawa J, Kuwata T, Shimoda T, Katayama H, Kushima R, Taniguchi H, Yoshikawa T, Boku N, Terashima M, Fukuda H, Sano T, Sasako M. Posttherapy topographical nodal status, ypN-site, predicts survival of patients who received neoadjuvant chemotherapy followed by curative surgical resection for non-type 4 locally advanced gastric cancer: supplementary analysis of JCOG1004-A. Gastric Cancer, 24:197-204, 2021
4. Tanaka K, Ogawa G, Mizusawa J, Kadota T, Nakamura K, Shimada Y, Hamaguchi T, Fujita S, Kitano S, Inomata M, Kanemitsu Y, Fukuda H. Second primary cancers and recurrence in patients after resection of colorectal cancer: An integrated analysis of trials by Japan Clinical Oncology Group: JCOG1702A. Jpn J Clin Oncol, 51:185-191, 2021
5. Hara H, Mizusawa J, Hironaka S, Kato K, Daiko H, Abe T, Nakamura K, Ando N, Kitagawa Y. Influence of preoperative chemotherapy-induced leukopenia on survival in patients with esophageal squamous cell carcinoma: exploratory analysis of JCOG9907. Esophagus, 18:41-48, 2021
6. Miyamoto K, Watanabe T, Wakabayashi M, Nakamura K, Watanabe Y, Maruyama D, Tobinai K, Tsukasaki K, Fukuda H. Comparison of the International Workshop Criteria and the Response Evaluation Criteria in Solid Tumors for indolent B-cell lymphoma. Int J Clin Oncol, 26:429-437, 2021
7. Nozaki M, Kagami Y, Machida R, Nakamura K, Ito Y, Nishimura Y, Teshima T, Saito Y, Nagata Y, Matsumoto Y, Akimoto T, Hiraoka M. Final analysis of a Multicenter Single-Arm Confirmatory Trial of hypofractionated whole breast irradiation after breast-conserving surgery in Japan: JCOG0906. Jpn J Clin Oncol, 51:865-872, 2021
8. Mizuno T, Kojima Y, Yonemori K, Yoshida H, Sugiura Y, Ohtake Y, Okuma HS, Nishikawa T, Tanioka M, Sudo K, Shimomura A, Noguchi E, Kato T, Shimoi T, Uno M, Ishikawa M, Fujiwara Y, Ohe Y, Tamura K. HER3 protein expression as a risk factor for post-operative recurrence in patients with early-stage adenocarcinoma and adenosquamous carcinoma of the cervix. Oncol Lett, 20:38, 2020
9. Mizuno T, Kojima Y, Yonemori K, Yoshida H, Sugiura Y, Ohtake Y, Okuma HS, Nishikawa T, Tanioka M, Sudo K, Shimomura A, Noguchi E, Kato T, Shimoi T, Uno M, Ishikawa M, Fujiwara Y, Ohe Y, Tamura K. Neoadjuvant chemotherapy promotes the expression of HER3 in patients with ovarian cancer. Oncol Lett, 20:336, 2020
10. Sato J, Satouchi M, Itoh S, Okuma Y, Niho S, Mizugaki H, Murakami H, Fujisaka Y, Kozuki T, Nakamura K, Nagasaka Y, Kawasaki M, Yamada T, Machida R, Kuchiba A, Ohe Y, Yamamoto N. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol, 21:843-850, 2020
11. Sato Y, Mizusawa J, Katayama H, Nakamura K, Fukagawa T, Katai H, Haruta S, Yamada M, Takagi M, Tamura S, Yoshimura T, Tokunaga M, Yoshikawa T, Boku N, Sano T, Sasako M, Terashima M. Diagnosis of invasion depth in resectable advanced gastric cancer for neoadjuvant chemotherapy: An exploratory analysis of Japan clinical oncology group study: JCOG1302A. Eur J Surg Oncol, 46:1074-1079, 2020
12. Miyamoto K, Wakabayashi M, Mizusawa J, Nakamura K, Katayama H, Higashi T, Inomata M, Kitano S, Fujita S, Kanemitsu Y, Fukuda H. Evaluation of the representativeness and generalizability of Japanese clinical trials for localized rectal/colon cancer: Comparing participants in the Japan Clinical Oncology Group study with patients in Japanese registries. Eur J Surg Oncol, 46:1642-1648, 2020
13. Hironaka S, Komori A, Machida R, Ito Y, Takeuchi H, Ogawa G, Kato K, Onozawa M, Minashi K, Yano T, Nakamura K, Tsushima T, Hara H, Nozaki I, Ura T, Chin K, Fukuda H, Kitagawa Y. The association of primary tumor site with acute adverse event and efficacy of definitive chemoradiotherapy for cStage II/III esophageal cancer: an exploratory analysis of JCOG0909. Esophagus, 17:417-424, 2020
14. Iwasa S, Okita N, Kuchiba A, Ogawa G, Kawasaki M, Nakamura K, Shoji H, Honma Y, Takashima A, Kato K, Hamaguchi T, Boku N, Yamada Y. Phase II study of lenvatinib for metastatic colorectal cancer refractory to standard chemotherapy: the LEMON study (NCCH1503). ESMO Open, 5:2020
15. Nakamura K, Maruyama D. What is the value of health-related quality of life in a negative superiority trial? Ann Oncol, 31:976-977, 2020
16. Okuma HS, Yonemori K, Narita SN, Sukigara T, Hirakawa A, Shimizu T, Shibata T, Kawai A, Yamamoto N, Nakamura K, Nishida T, Fujiwara Y. MASTER KEY Project: Powering Clinical Development for Rare Cancers Through a Platform Trial. Clin Pharmacol Ther, 108:596-605, 2020
17. Nishimura Y, Ishikura S, Shibata T, Kodaira T, Ito Y, Tsuchiya K, Murakami Y, Saitoh JI, Akimoto T, Nakata K, Yoshimura M, Teshima T, Toshiyasu T, Ota Y, Ishikawa K, Shimizu H, Minemura T, Nakamura K, Hiraoka M. A phase II study of adaptive two-step intensity-modulated radiation therapy (IMRT) with chemotherapy for loco-regionally advanced nasopharyngeal cancer (JCOG1015). Int J Clin Oncol, 25:1250-1259, 2020
18. Terada M, Nakamura K, Martinelli F, Pe M, Mizusawa J, Eba J, Fukuda H, Kiyota N, Gatellier L, Majima Y, Velikova G, Bottomley A. Results from a 1-day workshop on the assessment of quality of life in cancer patients: a joint initiative of the Japan Clinical Oncology Group and the European Organisation for Research and Treatment of Cancer. Jpn J Clin Oncol, 50:1333-1341, 2020
19. Nakamura K, Shibata T. Regulatory changes after the enforcement of the new Clinical Trials Act in Japan. Jpn J Clin Oncol, 50:399-404, 2020
