Center for Research Administration and Support (CRAS)
Teruhiko Yoshida, Masaki Shibatuji, Nobuko Ushirozawa, Sachie Senda, Kiyokazu Kawashima, Fumie Suzuki, Kiyoka Nagayama, Yuko Yamada, Yuichi Amanuma, Sawako Nakayama, Anne Mochizuki, Miko Ito, Mayumi Yoshida, Asuka Fujisaki, Masaki Yamamoto, Genta Ohno, Yukari Nakayama, Kyoko Chiba, Maho Muraoka, Hanae Takahashi, Kazunori Aoki, Kazuhiko Aoyagi, Taro Shibata, Aya Kuchiba, Junki Mizusawa, Masashi Wakabayashi, Gakuto Ogawa, Ryunosuke Machida, Ryo Sadachi, Chihiro Yaita, Yuki Konda, Kohei Uemura, Ryo Kitabayashi, Kanako Fuyama, Kenji Matsui, Tsunakuni Ikka, Noriko Yamashita, Waki Toya, Haruka Nakada, Tomoko Seki, Satoko Arai, Tomoko Ishida, Norie Enomoto, Yayoi Ofuji, Sanae Kawada, Kuniko Takahashi, Chiaki Nishikawa, Mariko Nishima, Yoko Yokota, Masahiko Ozaki, Yuki Harada, Rika Oumi, Hayato Kamata, Kaori Yanagisawa, Chiemi Notake, Seiko Kondou, Megumi Shimada, Fusako Tai, Madoka Nakayama
Introduction
The Center for Research Administration and Support (CRAS) was established on July 16, 2014. The starting members of the CRAS were approximately 160 staff, who together offered diverse functions and specialties, ranging from research fund administration, alliances with the private sector, intellectual property, clinical research coordinators and data managers, monitoring and audit, biostatistics support, offices for research ethics (IRB), and COI committees.
The background and the purpose of the creation of the CRAS was explained by Dr. Tomomitsu Hotta, the President of the National Cancer Center (NCC), in the NCC News 2014 Vol.5 No.3 (in Japanese). Briefly, since its foundation in 1962, the NCC has added several new segments and organizations to evolve as a comprehensive cancer center. Because each segment needed its own research infrastructure, support activities in the NCC had become fragmented and scattered with the possibility of gaps and redundancies. Dr. Hotta and his Strategic Planning Bureau assembled the “NCC New Vision” in 2014, in which he proposed integration and communication for various research support functions in the NCC. The CRAS was created in response to the 2014 Vision.
In 2015, the NCC Hospital (NCCH) and the NCC Hospital East (NCCHE) were certified as the Core Clinical Research Hospital under the Medical Care Act in August and September, respectively. As a result, the governance of the Research Coordination Division, Research Promotion Division and Regulatory Science Section of the CRAS has moved to the Clinical Research Support Offices, which belong to the common departments of each hospital. In January 2017, these divisions and section were officially separated from the CRAS in the NCC organization.
Another major reformation of the CRAS in 2017 was the establishment of the Bioethics Division by expanding the former Bioethics Section. The Clinical Trials Act was promulgated on April 14, 2017 and came into effect on April 1, 2018. Despite the general scarcity of human resources in the specialty field, the NCC has been endowed with strong staff, who have been contributing to work not just inside the NCC but also all over Japan with regard to research ethics-related issues including the implementation of the Clinical Trial Act. In 2018, the concept of the RA (research administrator) system in the NCC was discussed in the CRAS and updated to the NCC headquarters. In 2019, under the leadership of the new Chief of Bioethics Division, several important discussion and rule-making were done in collaboration with the researchers and staff in both campuses of the NCC.
In 2020, the CRAS continued to work in response to directions by the governments on the research governance, such as the establishment of the basic policy for the data sharing by the NCC. The CRAS led the coordination of various sections on both campuses to grasp more precise and comprehensive picture of the research-related funding and revenue of the NCC.
(Future prospects)
The NCC embarked on the new era under the leadership of a newly appointed President and Directors of the NCC Research Institute (NCCRI) and both hospitals (NCCH, NCCHE) on April 1, 2016. The Vision and high-priority research targets have been redefined, and re-organization is in progress in various parts of the NCC. However, the core concept of the CRAS stays unchanged to contribute to the NCC mission by promoting organic unity of the NCC as a whole. It is particularly important to respect differences in roles and characteristics of each NCC campus, Tsukiji and Kashiwa, to make the most of their strong points to maximize the research output of the NCC. Collaborations with the Clinical Research Support Offices of both hospitals remain crucial, and the CRAS aims to contribute to bringing the various sections together in the NCC.
1. Research Administration Division
1) Research Administration Section
The Research Administration Section is a central office in charge of various administrative work related to research funding including application and reporting. The major external funding sources of the NCC are competitive grants from the government and government-supported agencies, such as the Ministry of Health, Labour and Welfare (MHLW), the Japan Science and Technology Agency (JST), and the Japan Agency for Medical Research and Development (AMED).
This section also serves as an administrative office for the NCC Research and Development Fund, which is provided directly from the government to the NCC for fulfillment of its mission as the national core institute of cancer control. This section organized seminars regarding research funding and its rules to prevent financial misconduct.
(Future prospects)
The Guidelines for Managing and Auditing Public Research Funds at Research Institutes was updated by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in February 2014 and adopted by the MHLW in March 2014. This section serves as a compliance promotion office of the Guidelines and has established a new system for research fund administration, which is fully compliant with the new Guidelines.
2) Research Alliance Section and Intellectual Property Section
Research Alliance and Intellectual Property Section (RAIP) promotes adequate transitions of the NCC's research to industry by unitarily managing the collaborative research alliances and the utilization of intellectual properties (IPs).
1. Alliance with the private sector
The RAIP has been helping the NCC to establish worldwide comprehensive collaborative partnerships with major pharmaceutical companies and academic institutions. Currently, 11 comprehensive collaborative alliances are active between the NCC and industry partners (Fig. 1). The RAIP has also been supporting a nationwide genomic screening project (SCRUM-Japan “Cancer Genome Screening Project for Individualized Medicine in Japan”). More than 20 pharmaceutical companies have joined the project (Table 1). The collaborative researches and research funds that RAIP manages have been increasing each year (Fig. 2). In 2020, the number of collaborations was 528, and the amount of research funds reached 3.9 billion yen.
2. Intellectual property management
The RAIP encourages the NCC’s researchers to license-out IPs derived from their researches at early stages to partner with companies who can work together towards realization of their researches. For those IPs the NCC essentially bears the cost, the RAIP constantly reviews the value of the IPs and dismisses those which could not find sponsors within a certain period. Through the RAIP’s efforts to allocate the NCC’s resources to IPs with commercial value, the NCC has been successful in sustaining a positive turnout of IP management for years (Fig. 3).
Figure 1. Comprehensive Research Alliance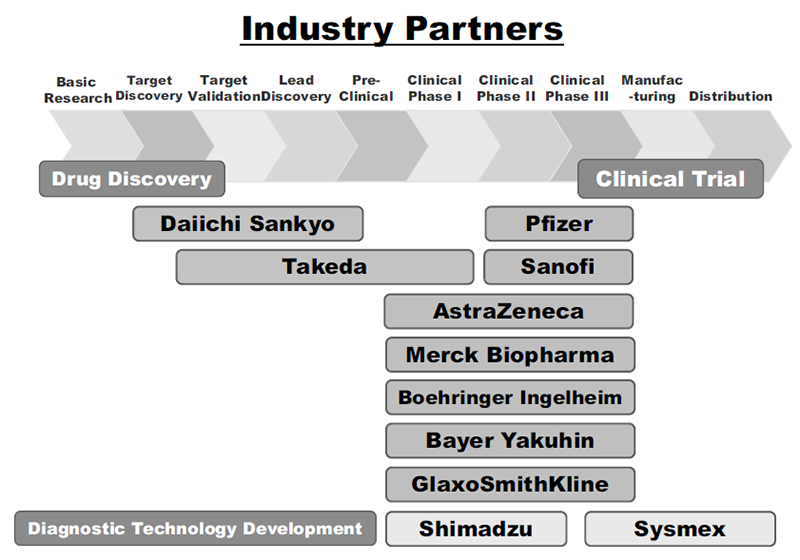

Figure 2. Collaborative Research Trends in the NCC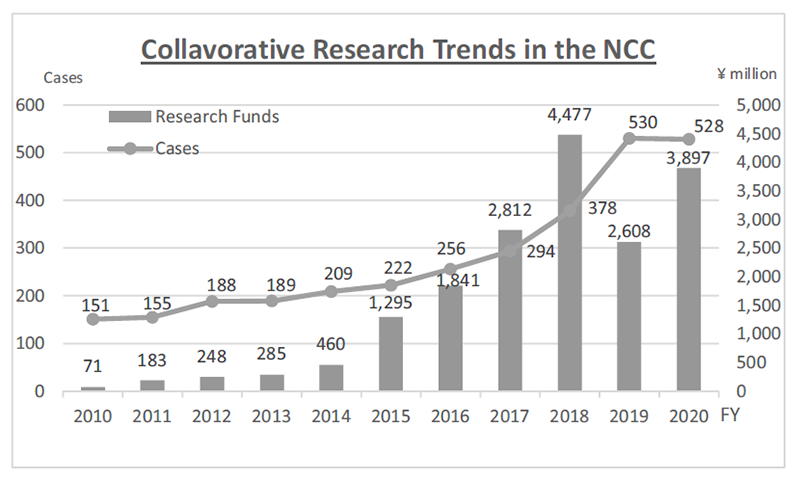

Figure 3. Licensing Trends in the NCC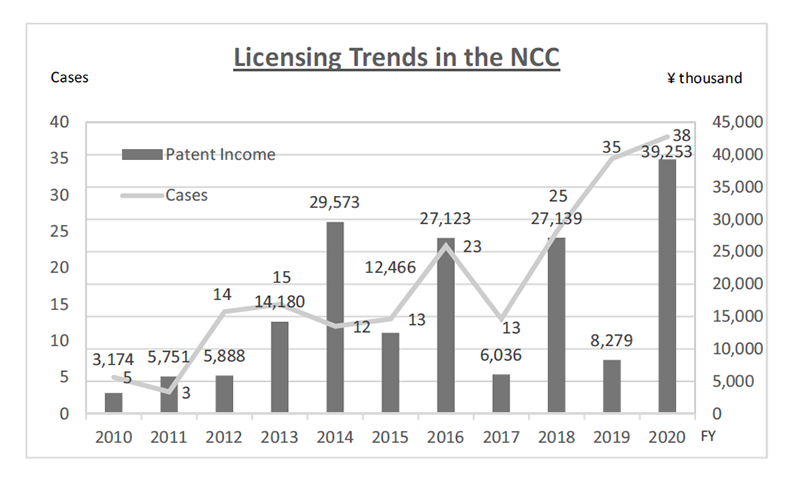

Table 1. Participating Companies in SCRUM-Japan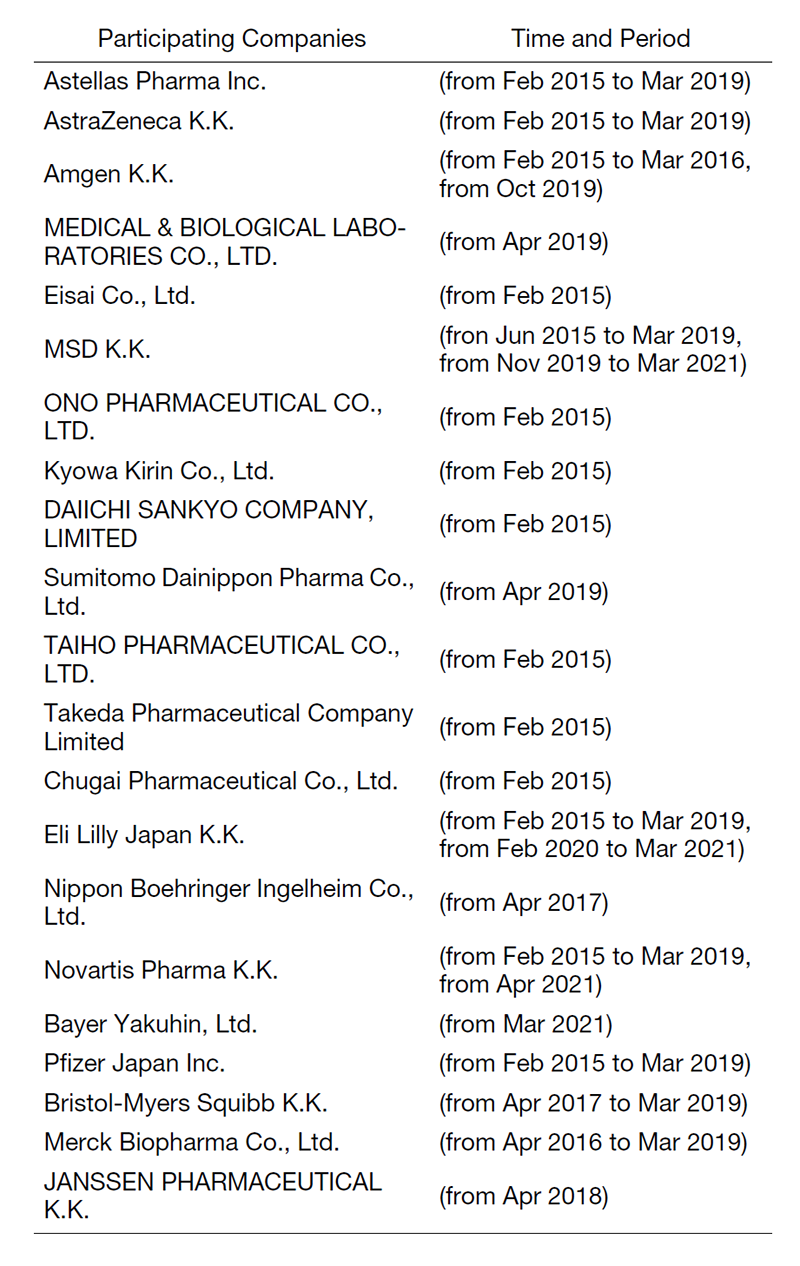

(Human resource development and education)
The RAIP hosts educational seminars on essential IP knowledge for NCC faculties at least twice a year. Members of the RAIP are encouraged to participate in seminars and conferences to update their knowledge on IP laws, regulations, and guidelines as well as scientific technologies, all of which are necessary to promote the academic-industrial alliances. From time to time, the RAIP utilizes consultations with experts, such as lawyers and patent attorneys, to solve problems and to gain practical experiences.
(Future prospects)
Under the NCC’s vision of “commitment for providing the best possible cancer treatment and care through relentless partnership with the community”, the RAIP will continue to support the creation of effective and innovative collaborative research frameworks which develop to fruitful research partnerships. The RAIP will continue to play key roles in assisting the NCC with making decisions on its IP management.
3) Research Administrators
Research administrators (RA) has supported to translate research outcomes into practical application based upon a comprehensive alliance with companies. As part of the Tsukiji Translational Research (TR) Board activity, RAs have conducted an informational meeting targeted at pharmaceutical companies to introduce the bioresources, technologies and TR supporting system in the NCC to promote co-clinical studies in cooperation with companies. RAs also helped the progress of 6NC cooperated research projects with Japan Health Research Promotion Bureau (JH).
(Future Prospects)
RAs promote acquisition of large competitive research funds through reinforcing the collaboration between organizations in the NCC, and also promote the translation of innovative research in the NCC to clinical diagnostic and therapeutic development and to patient care through several major mechanisms: the NCC Seeds Selection Committee, comprehensive alliances with leading companies, participation in the academic Drug Discovery Network, establishment of NCC spin-off venture companies, and cooperation between national centers and JH.
2. Biostatistics Division
Biostatistics Division has a role of responsibility in study design, analysis, interpretation and publication, especially in JCOG and EPOC clinical trials and the investigator-initiated clinical trials which are led by investigators in the NCC Hospitals. We have also committed to establish an infrastructure to support the clinical trials in the NCC Hospitals. Furthermore, we have been actively involved in collaborative relationships with the NCC Research Institute and the Center for Public Health Sciences.
We provided 13 introductory biostatistics lectures for investigators in the NCC to learn and review the elementary aspect of biostatistics. We had a cumulative total of 607 participants. In addition, we hosted a biostatistics lecture to cover the important biostatistical side of various application fields and a total of 94 investigators participated. The lectures are open to any applicants from external institutes.
Furthermore, we have provided biostatistical consultation and expertise, which supports NCC investigators working on basic, translational, clinical and epidemiological researches. We offered advice on 114 problems (63 in Tsukiji campus and 51 in Kashiwa campus) for which biostatistical consultation was requested from April 2020 to March 2021.
In addition, we provided an internship program for 17 graduate students from the graduate schools of the University of Tokyo (the Biostatistics and Bioinformatics Course, Graduate School of Interdisciplinary Information Studies).
(Future Prospects)
The NCC has a critical role in providing clinical service, education, conducting researches and making policy recommendations/proposals, all of which are required to make a decision on the basis of solid and scientific evidence from reliable data and information. The mission of the Biostatistics Division is to contribute to providing the best evidence and improving clinical practice and public health through the development and application of statistical methods. The Biostatistics Division is expanding on its independent and collaborative research with a range of areas, including prevention and policy recommendation/proposal, as well as treatment development. This year, we have made a particular effort to build effective relationships with the investigators at the NCC Research Institute and the Center for Public Health Sciences. Collaborative projects have been launched, including the evaluation and implementation of medical technology with artificial intelligence. Collaboration on epidemiologic studies have motivated to develop statistical methods to gain deeper insight into etiological mechanisms or public health impact. We are working on promoting cooperative framework with external experts in statistics/biostatistics. We are opening up a new methodological research area in which a mathematical approach will serve as a solid basis.
3. Bioethics Division
The Bioethics Division has a mission of providing information, education and training programs of research ethics including human subject protection, conflict of interests (COIs) management on clinical research, and the administration of institutional research ethics reviews boards (IRBs). In addition to this mission, each of the following three Sections under the Division has its own specific roles as described below.
1) Bioethics and Healthcare Law Section
The Bioethics and Healthcare Law Section (BHLS) provides research ethics consultation services (RECS) to researchers, research supporters, IRB members, and other concerned parties or groups of people. The RECS has an advisory function, which does not hold any compulsory or binding force and therefore differs from the roles of the IRBs, so as to give instructions and advice in terms of ethics on how to handle and solve ethical and sometimes legal issues arising in each human research study. The RECS therefore sometimes goes beyond regulatory compliance. In FY2020, the BHLS held 261 consultations (92 at Tsukiji campus, and 169 at Kashiwa campus), which involved issues regarding the Clinical Trial Act (3), governmental ethical guidelines (254), and others (4). In addition, the BHLS held several educational seminars for both NCC and non-NCC researchers and/or IRB staff.
(Future Prospects)
The BHLS will continue providing and promoting the RECS as well as offering educational materials of research ethics, so as to enhance ethical conducts of human subjects research. In FY2021 in particular, as the new governmental ethics guidelines are scheduled to be enforced at the end of June 2021, the BHLS will prepare itself to provide appropriate consultations conformable to the new guidelines.
2) Human Research Protection Section
The Human Research Protection Section (HRPS) serves as the administrative offices for the various types of IRBs for human subject research, including the certified review committees defined by the Clinical Trials Act (Act No. 16 of April 14, 2017).
The accomplishments of the reviews by the IRBs at the NCC in FY2020 included 1650 active research projects and 469 new research plans by NCC faculty members; while a total of 97 new research plans by researchers outside of the NCC were requested for review.
(Future Prospects)
The HRPS will continue working, in cooperation with the NCCH’s and the NCCHE’s Ethical Review Support Sections, to ameliorate the NCC’s whole ethics review processes to establish more appropriate and efficient ways. Particularly in FY2021, the HRPS will adjust and manage the institutional ethics review system for the new ethics guidelines which is scheduled to be enforced at the end of June, 2021.
3) COI Management Section
The COI Management Section (COIMS) is the administrative office of the COI Review Committee on Clinical Research at the NCC, in addition to responding to COI-related inquiries from researchers. The targets of the COI reviews are individual researchers and research projects using public research funds, but each member of various committees at the NCC also undergo the COI reviews. Specified clinical trials under Clinical Trials Act are not reviewed by the COI Review Committee, but the COIMS helps researchers of those trials to make the forms of COI management and check the facts of COI.
In FY2020, the COIMS managed 2502 researchers’ COI reviews in the 137 investigator-induced clinical trials under the Pharmaceutical Affairs Law, and 2331 researchers’ COI reviews in the 216 research projects under the non-binding governmental ethical guidelines; we also provided consultations for 12 inquiries from other departments at the NCC.
(Future Prospects)
The COIMS will continue to strive for efficient, high quality management of COIs, as well as proving the latest information on the COI standards for researchers and committee members. Particularly in FY2021, the COIMS will aim to consider the management of institutional COI and utilize the system in making forms of specified clinical trials under Clinical Trials Act for the convenience of researchers.
List of papers published in 2020
Journal
1. Murakami N, Mori T, Machida R, Kodaira T, Ito Y, Shikama N, Konishi K, Matsumoto Y, Murakami Y, Nakamura N, Yamashita H, Yorozu A, Yoshimura M, Inoue K, Nozaki M, Ishikura S, Itami J, Nishimura Y, Kagami Y. Prognostic Value of Epithelial Cell Adhesion Molecules in T1-2N0M0 Glottic Cancer. Laryngoscope, 131:1522-1527, 2021
2. Aokage K, Suzuki K, Wakabayashi M, Mizutani T, Hattori A, Fukuda H, Watanabe SI. Predicting pathological lymph node status in clinical stage IA peripheral lung adenocarcinoma. Eur J Cardiothorac Surg, 2021
3. Fujitani K, Nakamura K, Mizusawa J, Kuwata T, Shimoda T, Katayama H, Kushima R, Taniguchi H, Yoshikawa T, Boku N, Terashima M, Fukuda H, Sano T, Sasako M. Posttherapy topographical nodal status, ypN-site, predicts survival of patients who received neoadjuvant chemotherapy followed by curative surgical resection for non-type 4 locally advanced gastric cancer: supplementary analysis of JCOG1004-A. Gastric Cancer, 24:197-204, 2021
4. Hara H, Mizusawa J, Hironaka S, Kato K, Daiko H, Abe T, Nakamura K, Ando N, Kitagawa Y. Influence of preoperative chemotherapy-induced leukopenia on survival in patients with esophageal squamous cell carcinoma: exploratory analysis of JCOG9907. Esophagus, 18:41-48, 2021
5. Kadota T, Minashi K, Wakabayashi M, Yano T, Ezoe Y, Tsuchida T, Ono H, Iizuka T, Matsuura N, Oda I, Takizawa K, Katayama H, Fukuda H, Muto M. Diagnostic yield of conventional endoscopy with endoscopic ultrasonography for submucosal invasion of superficial esophageal squamous cell carcinoma: a post hoc analysis of multicenter prospective confirmatory study (JCOG0508). Esophagus, 18:604-611, 2021
6. Katayama H, Mizusawa J, Fukuda H, Nakamura S, Nakamura K, Saijo N, Yokoyama A, Ohe Y, Shinkai T, Nakagawa K, Abe T, Mitsuoka S, Okamoto H, Yamamoto N, Yoshioka H, Ando M, Tamura T, Takeda K. Prognostic impact of geriatric assessment in elderly patients with non-small cell lung cancer: an integrated analysis of two randomized phase III trials (JCOG1115-A). Jpn J Clin Oncol, 51:685-692, 2021
7. Tokunaga M, Kurokawa Y, Machida R, Sato Y, Takiguchi S, Doki Y, Yabusaki H, Watanabe M, Hato S, Nakamori M, Ito S, Yoshikawa T, Terashima M. Impact of postoperative complications on survival outcomes in patients with gastric cancer: exploratory analysis of a randomized controlled JCOG1001 trial. Gastric Cancer, 24:214-223, 2021
8. Inokuchi J, Kuroiwa K, Nishiyama H, Kojima T, Kakehi Y, Sugimoto M, Takenaka A, Fujimoto K, Yamaguchi R, Habuchi T, Hashine K, Mizusawa J, Eba J, Naito S. Significance of the timing of ureteral ligation on prognosis during radical nephroureterectomy for upper urinary tract urothelial cancer. Int J Urol, 28:208-214, 2021
9. Ioka T, Furuse J, Fukutomi A, Mizusawa J, Nakamura S, Hiraoka N, Ito Y, Katayama H, Ueno M, Ikeda M, Sugimori K, Okano N, Shimizu K, Yanagimoto H, Okusaka T, Ozaka M, Todaka A, Nakamori S, Tobimatsu K, Sata N, Kawashima Y, Hosokawa A, Yamaguchi T, Miyakawa H, Hara H, Mizuno N, Ishii H. Randomized phase II study of chemoradiotherapy with versus without induction chemotherapy for locally advanced pancreatic cancer: Japan Clinical Oncology Group trial, JCOG1106. Jpn J Clin Oncol, 51:235-243, 2021
10. Maruyama D, Iida S, Ogawa G, Fukuhara N, Seo S, Miyazaki K, Yoshimitsu M, Kuroda J, Tsukamoto N, Tsujimura H, Hangaishi A, Yamauchi T, Utsumi T, Mizuno I, Takamatsu Y, Nagata Y, Minauchi K, Ohtsuka E, Hanamura I, Yoshida S, Yamasaki S, Suehiro Y, Kamiyama Y, Tsukasaki K, Nagai H. Randomised phase II study to optimise melphalan, prednisolone, and bortezomib in untreated multiple myeloma (JCOG1105). Br J Haematol, 192:531-541, 2021
11. Miyamoto K, Watanabe T, Wakabayashi M, Nakamura K, Watanabe Y, Maruyama D, Tobinai K, Tsukasaki K, Fukuda H. Comparison of the International Workshop Criteria and the Response Evaluation Criteria in Solid Tumors for indolent B-cell lymphoma. Int J Clin Oncol, 26:429-437, 2021
12. Ohmachi K, Kinoshita T, Tobinai K, Ogawa G, Mizutani T, Yamauchi N, Fukuhara N, Uchida T, Yamamoto K, Miyazaki K, Tsukamoto N, Iida S, Utsumi T, Yoshida I, Imaizumi Y, Tokunaga T, Yoshida S, Masaki Y, Murayama T, Yakushijin Y, Suehiro Y, Nosaka K, Dobashi N, Kuroda J, Takamatsu Y, Maruyama D, Ando K, Ishizawa K, Ogura M, Yoshino T, Hotta T, Tsukasaki K, Nagai H. A randomized phase 2/3 study of R-CHOP vs CHOP combined with dose-dense rituximab for DLBCL: the JCOG0601 trial. Blood Adv, 5:984-993, 2021
13. Onda T, Tanaka YO, Kitai S, Manabe T, Ishikawa M, Hasumi Y, Miyamoto K, Ogawa G, Satoh T, Saito T, Kasamatsu T, Nakanishi T. Stage III disease of ovarian, tubal and peritoneal cancers can be accurately diagnosed with pre-operative CT. Japan Clinical Oncology Group Study JCOG0602. Jpn J Clin Oncol, 51:205-212, 2021
14. Tanaka K, Ogawa G, Mizusawa J, Kadota T, Nakamura K, Shimada Y, Hamaguchi T, Fujita S, Kitano S, Inomata M, Kanemitsu Y, Fukuda H. Second primary cancers and recurrence in patients after resection of colorectal cancer: An integrated analysis of trials by Japan Clinical Oncology Group: JCOG1702A. Jpn J Clin Oncol, 51:185-191, 2021
15. Imamura Y, Toihata T, Haraguchi I, Ogata Y, Takamatsu M, Kuchiba A, Tanaka N, Gotoh O, Mori S, Nakashima Y, Oki E, Mori M, Oda Y, Taguchi K, Yamamoto M, Morita M, Yoshida N, Baba H, Mine S, Nunobe S, Sano T, Noda T, Watanabe M. Immunogenic characteristics of microsatellite instability-low esophagogastric junction adenocarcinoma based on clinicopathological, molecular, immunological and survival analyses. Int J Cancer, 148:1260-1275, 2021
16. Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito S, Kaji M, Kimura Y, Hirao M, Yamada M, Kurita A, Takagi M, Lee SW, Takagane A, Yabusaki H, Hihara J, Boku N, Sano T, Sasako M. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer, 24:492-502, 2021
17. Takizawa K, Ono H, Hasuike N, Takashima A, Minashi K, Boku N, Kushima R, Katayama H, Ogawa G, Fukuda H, Fujisaki J, Oda I, Yano T, Hori S, Doyama H, Hirasawa K, Yamamoto Y, Ishihara R, Tanabe S, Niwa Y, Nakagawa M, Terashima M, Muto M. A nonrandomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010). Gastric Cancer, 24:479-491, 2021
18. Yuwaki K, Kuchiba A, Otsuki A, Odawara M, Okuhara T, Ishikawa H, Inoue M, Tsugane S, Shimazu T. Effectiveness of a Cancer Risk Prediction Tool on Lifestyle Habits: A Randomized Controlled Trial. Cancer Epidemiol Biomarkers Prev, 30:1063-1071, 2021
19. Fujiwara Y, Kuchiba A, Koyama T, Machida R, Shimomura A, Kitano S, Shimizu T, Yamamoto N. Infection risk with PI3K-AKT-mTOR pathway inhibitors and immune checkpoint inhibitors in patients with advanced solid tumours in phase I clinical trials. ESMO Open, 5:2020
20. Tsukamoto S, Fujita S, Ota M, Mizusawa J, Shida D, Kanemitsu Y, Ito M, Shiomi A, Komori K, Ohue M, Akazai Y, Shiozawa M, Yamaguchi T, Bando H, Tsuchida A, Okamura S, Akagi Y, Takiguchi N, Saida Y, Akasu T, Moriya Y. Long-term follow-up of the randomized trial of mesorectal excision with or without lateral lymph node dissection in rectal cancer (JCOG0212). Br J Surg, 107:586-594, 2020
21. Natsume A, Aoki K, Ohka F, Maeda S, Hirano M, Adilijiang A, Motomura K, Sumi M, Nishikawa R, Narita Y, Muragaki Y, Maruyama T, Ito T, Beppu T, Nakamura H, Kayama T, Sato S, Nagane M, Mishima K, Nakasu Y, Kurisu K, Yamasaki F, Sugiyama K, Onishi T, Iwadate Y, Terasaki M, Kobayashi H, Matsumura A, Ishikawa E, Sasaki H, Mukasa A, Matsuo T, Hirano H, Kumabe T, Shinoura N, Hashimoto N, Aoki T, Asai A, Abe T, Yoshino A, Arakawa Y, Asano K, Yoshimoto K, Shibui S, Okuno Y, Wakabayashi T. Genetic analysis in patients with newly diagnosed glioblastomas treated with interferon-beta plus temozolomide in comparison with temozolomide alone. J Neurooncol, 148:17-27, 2020
22. Onda T, Satoh T, Ogawa G, Saito T, Kasamatsu T, Nakanishi T, Mizutani T, Takehara K, Okamoto A, Ushijima K, Kobayashi H, Kawana K, Yokota H, Takano M, Kanao H, Watanabe Y, Yamamoto K, Yaegashi N, Kamura T, Yoshikawa H. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur J Cancer, 130:114-125, 2020
23. Sato J, Satouchi M, Itoh S, Okuma Y, Niho S, Mizugaki H, Murakami H, Fujisaka Y, Kozuki T, Nakamura K, Nagasaka Y, Kawasaki M, Yamada T, Machida R, Kuchiba A, Ohe Y, Yamamoto N. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol, 21:843-850, 2020
24. Sato Y, Mizusawa J, Katayama H, Nakamura K, Fukagawa T, Katai H, Haruta S, Yamada M, Takagi M, Tamura S, Yoshimura T, Tokunaga M, Yoshikawa T, Boku N, Sano T, Sasako M, Terashima M. Diagnosis of invasion depth in resectable advanced gastric cancer for neoadjuvant chemotherapy: An exploratory analysis of Japan clinical oncology group study: JCOG1302A. Eur J Surg Oncol, 46:1074-1079, 2020
25. Yokomizo A, Wakabayashi M, Satoh T, Hashine K, Inoue T, Fujimoto K, Egawa S, Habuchi T, Kawashima K, Ishizuka O, Shinohara N, Sugimoto M, Yoshino Y, Nihei K, Fukuda H, Tobisu KI, Kakehi Y, Naito S. Salvage Radiotherapy Versus Hormone Therapy for Prostate-specific Antigen Failure After Radical Prostatectomy: A Randomised, Multicentre, Open-label, Phase 3 Trial (JCOG0401)(†). Eur Urol, 77:689-698, 2020
26. Kadota T, Ikematsu H, Sasaki T, Saito Y, Ito M, Mizutani T, Ogawa G, Shitara K, Ito Y, Kushima R, Kanemitsu Y, Muto M. Protocol for a single-arm confirmatory trial of adjuvant chemoradiation for patients with high-risk rectal submucosal invasive cancer after local resection: Japan Clinical Oncology Group Study JCOG1612 (RESCUE study). BMJ Open, 10:e034947, 2020
27. Mori M, Morita T, Matsuda Y, Yamada H, Kaneishi K, Matsumoto Y, Matsuo N, Odagiri T, Aruga E, Watanabe H, Tatara R, Sakurai H, Kimura A, Katayama H, Suga A, Nishi T, Shirado AN, Watanabe T, Kuchiba A, Yamaguchi T, Iwase S. How successful are we in relieving terminal dyspnea in cancer patients? A real-world multicenter prospective observational study. Support Care Cancer, 28:3051-3060, 2020
28. Nakajima TE, Yamaguchi K, Boku N, Hyodo I, Mizusawa J, Hara H, Nishina T, Sakamoto T, Shitara K, Shinozaki K, Katayama H, Nakamura S, Muro K, Terashima M. Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer, 23:677-688, 2020
29. Nishimura Y, Ishikura S, Shibata T, Kodaira T, Ito Y, Tsuchiya K, Murakami Y, Saitoh JI, Akimoto T, Nakata K, Yoshimura M, Teshima T, Toshiyasu T, Ota Y, Ishikawa K, Shimizu H, Minemura T, Nakamura K, Hiraoka M. A phase II study of adaptive two-step intensity-modulated radiation therapy (IMRT) with chemotherapy for loco-regionally advanced nasopharyngeal cancer (JCOG1015). Int J Clin Oncol, 25:1250-1259, 2020
30. Oashi K, Shibata T, Namikawa K, Takahashi A, Yokota K, Nakano E, Teramoto Y, Tsutsumida A, Maeda T, Yamazaki N. A single-arm confirmatory trial of pazopanib in patients with paclitaxel-pretreated primary cutaneous angiosarcoma: Japan Clinical Oncology Group study (JCOG1605, JCOG-PCAS protocol). BMC Cancer, 20:652, 2020
31. Iwasa S, Okita N, Kuchiba A, Ogawa G, Kawasaki M, Nakamura K, Shoji H, Honma Y, Takashima A, Kato K, Hamaguchi T, Boku N, Yamada Y. Phase II study of lenvatinib for metastatic colorectal cancer refractory to standard chemotherapy: the LEMON study (NCCH1503). ESMO Open, 5:2020
32. Kadota T, Tsukada Y, Ito M, Katayama H, Mizusawa J, Nakamura N, Ito Y, Bando H, Ando M, Onaya H, Fukuda H, Kanemitsu Y. A phase III randomized controlled trial comparing surgery plus adjuvant chemotherapy with preoperative chemoradiotherapy followed by surgery plus adjuvant chemotherapy for locally recurrent rectal cancer: Japan Clinical Oncology Group study JCOG1801 (RC-SURVIVE study). Jpn J Clin Oncol, 50:953-957, 2020
33. Kagami Y, Yamamoto K, Shibata T, Tobinai K, Imaizumi Y, Uchida T, Shimada K, Minauchi K, Fukuhara N, Kobayashi H, Yamauchi N, Tsujimura H, Hangaishi A, Tominaga R, Suehiro Y, Yoshida S, Inoue Y, Suzuki S, Tokuhira M, Kusumoto S, Kuroda J, Yakushijin Y, Takamatsu Y, Kubota Y, Nosaka K, Morishima S, Nakamura S, Ogura M, Maruyama D, Hotta T, Morishima Y, Tsukasaki K, Nagai H. R-CHOP-14 versus R-CHOP-14/CHASER for upfront autologous transplantation in diffuse large B-cell lymphoma: JCOG0908 study. Cancer Sci, 111:3770-3779, 2020
34. Miyamoto K, Wakabayashi M, Mizusawa J, Nakamura K, Katayama H, Higashi T, Inomata M, Kitano S, Fujita S, Kanemitsu Y, Fukuda H. Evaluation of the representativeness and generalizability of Japanese clinical trials for localized rectal/colon cancer: Comparing participants in the Japan Clinical Oncology Group study with patients in Japanese registries. Eur J Surg Oncol, 46:1642-1648, 2020
35. Shimoyama R, Hijioka S, Mizuno N, Ogawa G, Kataoka T, Katayama H, Machida N, Honma Y, Boku N, Hamaguchi T, Fukuda H, Terashima M, Kanemitsu Y, Furuse J. Study protocol for a multi-institutional randomized phase III study comparing combined everolimus plus lanreotide therapy and everolimus monotherapy in patients with unresectable or recurrent gastroenteropancreatic neuroendocrine tumors; Japan Clinical Oncology Group Study JCOG1901 (STARTER-NET study). Pancreatology, 20:1183-1188, 2020
36. Shimoyama R, Tsutani Y, Wakabayashi M, Katayama H, Fukuda H, Suzuki K, Watanabe SI. A multi-institutional randomized phase III trial comparing anatomical segmentectomy and wedge resection for clinical stage IA non-small cell lung cancer in high-risk operable patients: Japan Clinical Oncology Group Study JCOG1909 (ANSWER study). Jpn J Clin Oncol, 50:1209-1213, 2020
37. Tanaka K, Tsutani Y, Wakabayashi M, Mizutani T, Aokage K, Miyata Y, Kuroda H, Saji H, Watanabe SI, Okada M. Sublobar resection versus lobectomy for patients with resectable stage I non-small cell lung cancer with idiopathic pulmonary fibrosis: a phase III study evaluating survival (JCOG1708, SURPRISE). Jpn J Clin Oncol, 50:1076-1079, 2020
38. Hironaka S, Komori A, Machida R, Ito Y, Takeuchi H, Ogawa G, Kato K, Onozawa M, Minashi K, Yano T, Nakamura K, Tsushima T, Hara H, Nozaki I, Ura T, Chin K, Fukuda H, Kitagawa Y. The association of primary tumor site with acute adverse event and efficacy of definitive chemoradiotherapy for cStage II/III esophageal cancer: an exploratory analysis of JCOG0909. Esophagus, 17:417-424, 2020
39. Iwasaki M, Budhathoki S, Yamaji T, Tanaka-Mizuno S, Kuchiba A, Sawada N, Goto A, Shimazu T, Inoue M, Tsugane S. Inclusion of a gene-environment interaction between alcohol consumption and the aldehyde dehydrogenase 2 genotype in a risk prediction model for upper aerodigestive tract cancer in Japanese men. Cancer Sci, 111:3835-3844, 2020
40. Sato Y, Yamada T, Yoshikawa T, Machida R, Mizusawa J, Katayama H, Tokunaga M, Boku N, Terashima M. Randomized controlled Phase III trial to evaluate omentum preserving gastrectomy for patients with advanced gastric cancer (JCOG1711, ROAD-GC). Jpn J Clin Oncol, 50:1321-1324, 2020
41. Arita H, Matsushita Y, Machida R, Yamasaki K, Hata N, Ohno M, Yamaguchi S, Sasayama T, Tanaka S, Higuchi F, Iuchi T, Saito K, Kanamori M, Matsuda KI, Miyake Y, Tamura K, Tamai S, Nakamura T, Uda T, Okita Y, Fukai J, Sakamoto D, Hattori Y, Pareira ES, Hatae R, Ishi Y, Miyakita Y, Tanaka K, Takayanagi S, Otani R, Sakaida T, Kobayashi K, Saito R, Kurozumi K, Shofuda T, Nonaka M, Suzuki H, Shibuya M, Komori T, Sasaki H, Mizoguchi M, Kishima H, Nakada M, Sonoda Y, Tominaga T, Nagane M, Nishikawa R, Kanemura Y, Kuchiba A, Narita Y, Ichimura K. TERT promoter mutation confers favorable prognosis regardless of 1p/19q status in adult diffuse gliomas with IDH1/2 mutations. Acta Neuropathol Commun, 8:201, 2020
42. Suzuki K, Watanabe SI, Wakabayashi M, Saji H, Aokage K, Moriya Y, Yoshino I, Tsuboi M, Nakamura S, Nakamura K, Mitsudomi T, Asamura H. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg, 2020
43. Kenmotsu H, Niho S, Tsuboi M, Wakabayashi M, Ishii G, Nakagawa K, Daga H, Tanaka H, Saito H, Aokage K, Takahashi T, Menju T, Kasai T, Yoshino I, Minato K, Okada M, Eba J, Asamura H, Ohe Y, Watanabe SI. Randomized Phase III Study of Irinotecan Plus Cisplatin Versus Etoposide Plus Cisplatin for Completely Resected High-Grade Neuroendocrine Carcinoma of the Lung: JCOG1205/1206. J Clin Oncol, 38:4292-4301, 2020
44. Kitamura H, Hinotsu S, Tsukamoto T, Shibata T, Mizusawa J, Kobayashi T, Miyake M, Nishiyama N, Kojima T, Nishiyama H. Effect of neoadjuvant chemotherapy on health-related quality of life in patients with muscle-invasive bladder cancer: results from JCOG0209, a randomized phase III study. Jpn J Clin Oncol, 50:1464-1469, 2020
45. Kunitoh H, Tsuboi M, Wakabayashi M, Okada M, Suzuki K, Watanabe S, Asamura H, Fukuda H, Shibata T, Kazato T, Mizutani T, Eba J. A phase III study of adjuvant chemotherapy in patients with completely resected, node-negative non-small cell lung cancer (JCOG 0707). JTCVS Open, 4:90-102, 2020
46. Kawai A, Higashi T, Shibata T, Yoshida A, Katoh Y, Fujiwara Y, Nishida T. Rare cancers in Japan: definition, clinical features and future perspectives. Jpn J Clin Oncol, 50:970-975, 2020
