Annual Report 2020
Division of Cancer Immunology
Hiroyoshi Nishikawa, Yuka Maeda, Keisuke Watanabe, Hitomi Nishinakamura, Kota Itahashi, Priya Saju, Kana Kusaba, Kenta Nakama, Ai Sasaki, Takeru Naito, Yuki Ishige, Sakthisri Krishnamurthy, Prakhongcheep Ornjira, Nanae Shimoyama
Introduction
Cancer immunotherapies, such as immune checkpoint inhibitors (ICIs) or gene modified adoptive cell therapies, including chimeric antigen receptor T (CAR-T) cells or T-cell receptor T (TCR-T) cells, have shown significant efficacy in some patients. However, not a few patients experience relapse or eventually develop resistant disease. Cancers often create complex immune suppressive mechanisms that can impair host immune reaction, thereby canceling the efficacy of ICIs or adoptive transferred anti-tumor immune cells. Therefore, it is an urgent demand to elucidate the nature of cancer immunology, and to identify mechanisms of resistance and precise biomarkers to predict its efficacy for clinical practice.
The Team and What We Do
We have been intensively collecting clinical samples from cancer patients treated with immunotherapies and investigating the mechanisms of cancer immunology using multi-omics analysis, including flow cytometry, mass cytometry, single-cell transcriptome analysis, proteomics, and metabolomics (Figure 1). In addition, based on our findings, we are developing the first-in class gene-modified cell-based immunotherapies using CAR-T cell or TCR-T cell platforms (Figure 2).
Figure 1. Investigation of the dynamic immune state in cancer patients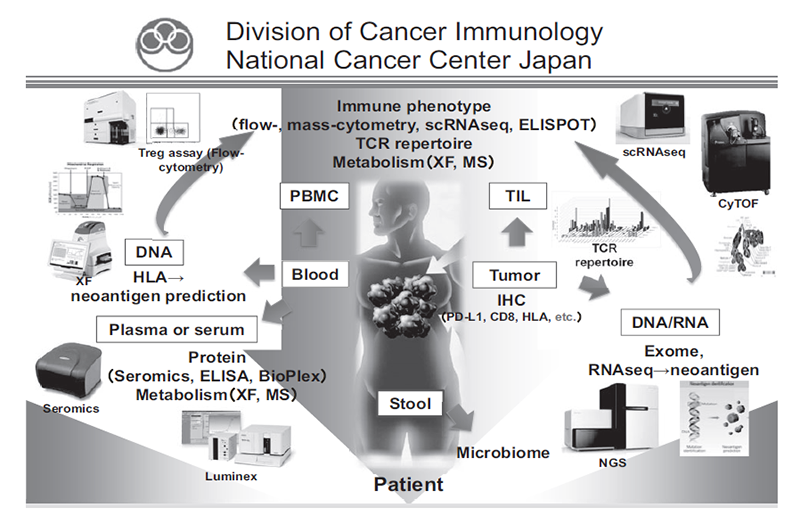

Figure 2. Development of novel CAR-T therapies and plan to perform clinical trial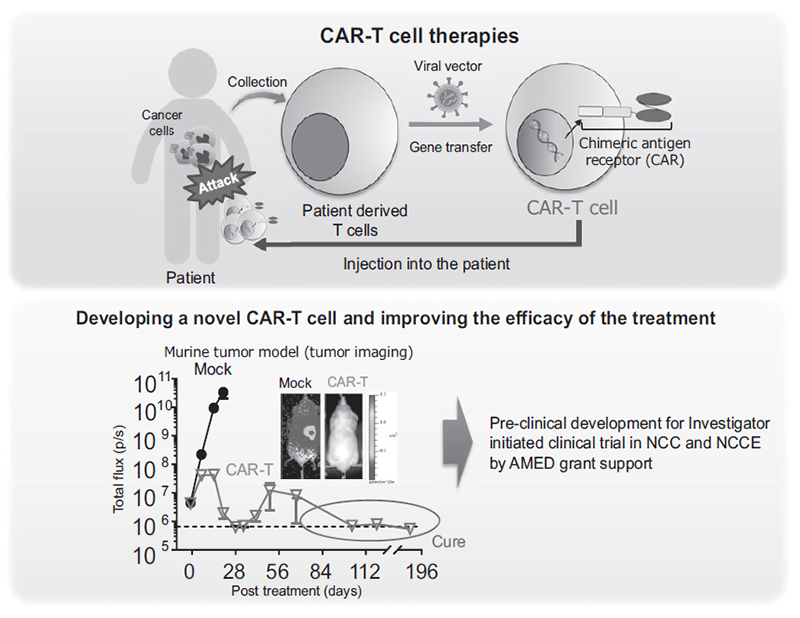

Research activities
We have identified multiple mechanisms that impair the efficacy of cancer immunotherapies using patient derived clinical samples pre- and post-treatment of ICIs. In addition, we have revealed metabolic features of regulatory T cells (Tregs) that can survive in a low-nutrient tumor microenvironment and suppress host immune reaction to the tumors. Interestingly, some tumors create features that metabolically support Treg survival in TME to evade host immune systems. For further analysis of interaction of immune cells with tumors and TME, we have established mouse models that enable three-dimensional kinetic analysis of immune cells by rendering mouse tissues transparent. Using this system, we have started to elucidate the kinetics of immune cells during immunotherapy. We are also developing novel CAR-T cells that overcome multiple immunosuppressive factors; to improve the treatment outcome of adoptive cell therapies in solid tumors, we are preparing for an investigator-initiated clinical trial.
Clinical trials
We have collaborated with several pharmaceutical companies in their clinical trials to elucidate predictive biomarkers and immune status in the tumor microenvironment using our multi-omics immune analysis system.
Education
Our division is hosting graduate students from universities with partnership agreements and young residents from the National Cancer Center Hospital for training in translational research. We strongly support their career development, and many alumni are leading the immunology field in a variety of posts in the clinic, academia, or corporations.
Future Prospects
We will further investigate the mechanisms involved in tumor immune suppression or tumor immune evasion in various cancers with a broad view into such areas as immunology and genetics, using new approaches including special and dynamic analysis of the transcriptome or immune cell phenotypes and transparent mouse tissue models. Our findings will help establish novel therapeutic approaches to overcome resistance and to identify precise biomarkers in immune therapies.
List of papers published in 2020
Journal
1. Arakawa A, Ichikawa H, Kubo T, Motoi N, Kumamoto T, Nakajima M, Yonemori K, Noguchi E, Sunami K, Shiraishi K, Kakishima H, Yoshida H, Hishiki T, Kawakubo N, Kuroda T, Kiyokawa T, Yamada K, Yanaihara N, Takahashi K, Okamoto A, Hirabayashi S, Hasegawa D, Manabe A, Ono K, Matsuoka M, Arai Y, Togashi Y, Shibata T, Nishikawa H, Aoki K, Yamamoto N, Kohno T, Ogawa C. Vaginal Transmission of Cancer from Mothers with Cervical Cancer to Infants. N Engl J Med, 384:42-50, 2021
2. Kumagai S, Koyama S, Nishikawa H. Antitumour immunity regulated by aberrant ERBB family signalling. Nat Rev Cancer, 21:181-197, 2021
3. Inamori K, Togashi Y, Fukuoka S, Akagi K, Ogasawara K, Irie T, Motooka D, Kobayashi Y, Sugiyama D, Kojima M, Shiiya N, Nakamura S, Maruyama S, Suzuki Y, Ito M, Nishikawa H. Importance of lymph node immune responses in MSI-H/dMMR colorectal cancer. JCI Insight, 6:2021
4. Seo W, Jerin C, Nishikawa H. Transcriptional regulatory network for the establishment of CD8+ T cell exhaustion. Exp Mol Med, 53:202-209, 2021
5. Kashima Y, Togashi Y, Fukuoka S, Kamada T, Irie T, Suzuki A, Nakamura Y, Shitara K, Minamide T, Yoshida T, Taoka N, Kawase T, Wada T, Inaki K, Chihara M, Ebisuno Y, Tsukamoto S, Fujii R, Ohashi A, Suzuki Y, Tsuchihara K, Nishikawa H, Doi T. Potentiality of multiple modalities for single-cell analyses to evaluate the tumor microenvironment in clinical specimens. Sci Rep, 11:341, 2021
6. Nozaki K, Fujioka Y, Sugiyama D, Ishikawa J, Iida M, Shibata M, Kosugi S, Nishikawa H, Shibayama H. Flow cytometry analysis of peripheral Tregs in patients with multiple myeloma under lenalidomide maintenance. Int J Hematol, 113:772-774, 2021
7. Chen S, Kiguchi T, Nagata Y, Tamai Y, Ikeda T, Kajiya R, Ono T, Sugiyama D, Nishikawa H, Akatsuka Y. A simple method to distinguish residual elotuzumab from monoclonal paraprotein in immunofixation assays for multiple myeloma patients. Int J Hematol, 113:473-479, 2021
8. Watanabe S, Goto Y, Yasuda H, Kohno T, Motoi N, Ohe Y, Nishikawa H, Kobayashi SS, Kuwano K, Togashi Y. HSP90 inhibition overcomes EGFR amplification-induced resistance to third-generation EGFR-TKIs. Thorac Cancer, 12:631-642, 2021
9. Muramatsu T, Noguchi T, Sugiyama D, Kanada Y, Fujimaki K, Ito S, Gotoh M, Nishikawa H. Newly emerged immunogenic neoantigens in established tumors enable hosts to regain immunosurveillance in a T-cell-dependent manner. Int Immunol, 33:39-48, 2021
10. Bando H, Kotani D, Tsushima T, Hara H, Kadowaki S, Kato K, Chin K, Yamaguchi K, Kageyama SI, Hojo H, Nakamura M, Tachibana H, Wakabayashi M, Fukutani M, Togashi Y, Fuse N, Nishikawa H, Kojima T. TENERGY: multicenter phase II study of Atezolizumab monotherapy following definitive Chemoradiotherapy with 5-FU plus Cisplatin in patients with unresectable locally advanced esophageal squamous cell carcinoma. BMC Cancer, 20:336, 2020
11. Kawazoe A, Kuboki Y, Shinozaki E, Hara H, Nishina T, Komatsu Y, Yuki S, Wakabayashi M, Nomura S, Sato A, Kuwata T, Kawazu M, Mano H, Togashi Y, Nishikawa H, Yoshino T. Multicenter Phase I/II Trial of Napabucasin and Pembrolizumab in Patients with Metastatic Colorectal Cancer (EPOC1503/SCOOP Trial). Clin Cancer Res, 26:5887-5894, 2020
12. Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, Sato E, Kuwata T, Kinoshita T, Yamamoto M, Nomura S, Tsukamoto T, Mano H, Shitara K, Nishikawa H. An Oncogenic Alteration Creates a Microenvironment that Promotes Tumor Progression by Conferring a Metabolic Advantage to Regulatory T Cells. Immunity, 53:187-203.e8, 2020
13. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y, Matsui S, Shibahara T, Yamashita Y, Irie T, Tsuge A, Fukuoka S, Kawazoe A, Udagawa H, Kirita K, Aokage K, Ishii G, Kuwata T, Nakama K, Kawazu M, Ueno T, Yamazaki N, Goto K, Tsuboi M, Mano H, Doi T, Shitara K, Nishikawa H. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol, 21:1346-1358, 2020
14. Umemoto K, Togashi Y, Arai Y, Nakamura H, Takahashi S, Tanegashima T, Kato M, Nishikawa T, Sugiyama D, Kojima M, Gotohda N, Kuwata T, Ikeda M, Shibata T, Nishikawa H. The potential application of PD-1 blockade therapy for early-stage biliary tract cancer. Int Immunol, 32:273-281, 2020
15. Nagasaki J, Togashi Y, Sugawara T, Itami M, Yamauchi N, Yuda J, Sugano M, Ohara Y, Minami Y, Nakamae H, Hino M, Takeuchi M, Nishikawa H. The critical role of CD4+ T cells in PD-1 blockade against MHC-II-expressing tumors such as classic Hodgkin lymphoma. Blood Adv, 4:4069-4082, 2020
16. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol, 38:2053-2061, 2020
17. Minoura K, Abe K, Maeda Y, Nishikawa H, Shimamura T. CYBERTRACK2.0: zero-inflated model-based cell clustering and population tracking method for longitudinal mass cytometry data. Bioinformatics, 2020
18. Abe K, Minoura K, Maeda Y, Nishikawa H, Shimamura T. Model-based clustering for flow and mass cytometry data with clinical information. BMC Bioinformatics, 21:393, 2020
19. Murate K, Maeda K, Nakamura M, Sugiyama D, Wada H, Yamamura T, Sawada T, Mizutani Y, Ishikawa T, Furukawa K, Ohno E, Honda T, Kawashima H, Miyahara R, Ishigami M, Nishikawa H, Fujishiro M. Endoscopic Activity and Serum TNF-α Level at Baseline Are Associated With Clinical Response to Ustekinumab in Crohn's Disease Patients. Inflamm Bowel Dis, 26:1669-1681, 2020
20. Kelkka T, Savola P, Bhattacharya D, Huuhtanen J, Lönnberg T, Kankainen M, Paalanen K, Tyster M, Lepistö M, Ellonen P, Smolander J, Eldfors S, Yadav B, Khan S, Koivuniemi R, Sjöwall C, Elo LL, Lähdesmäki H, Maeda Y, Nishikawa H, Leirisalo-Repo M, Sokka-Isler T, Mustjoki S. Adult-Onset Anti-Citrullinated Peptide Antibody-Negative Destructive Rheumatoid Arthritis Is Characterized by a Disease-Specific CD8+ T Lymphocyte Signature. Front Immunol, 11:578848, 2020
21. Sakai T, Terakura S, Miyao K, Okuno S, Adachi Y, Umemura K, Julamanee J, Watanabe K, Hamana H, Kishi H, Leitner J, Steinberger P, Nishida T, Murata M, Kiyoi H. Artificial T Cell Adaptor Molecule-Transduced TCR-T Cells Demonstrated Improved Proliferation Only When Transduced in a Higher Intensity. Mol Ther Oncolytics, 18:613-622, 2020
