Annual Report 2020
Laboratory of Molecular Carcinogenesis
Naoto Tsuchiya, Yuko Fujiwara, Takahiro Shirai, Yuma Takamoto
Introduction
To develop new strategies for cancer therapy and diagnosis, understanding molecular networks, formed intercellularly and intracellularly, is a major theme to be challenged. Our laboratory has been focusing on the biological functions of non-coding RNAs (ncRNAs). microRNAs, a class of ncRNAs, are known to be strong regulators of intracellular and intercellular connection of molecular networks. We have been extensively analyzing tumor-related miRNAs to find intracellular networks required for carcinogenesis, and have a major interest in the extracellular miRNAs as regulators for cancer cells to adapt to their microenvironment.
Research activities
1. Regulation of miRNA activity by the structural variant of miRNA and application in diagnostic biomarker
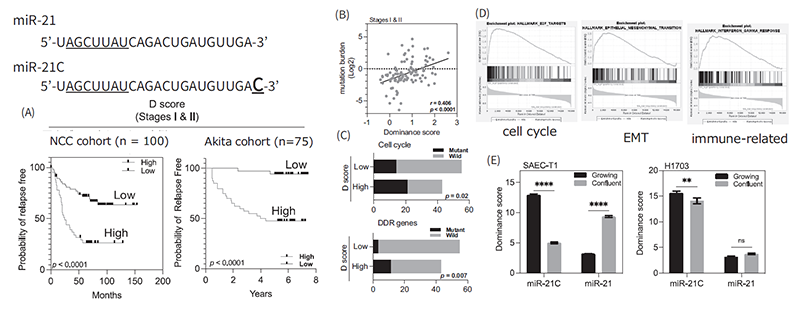
MicroRNAs (miRNA) function as a regulator of gene expression enabling to fine-tune of the intracellular molecular networks and are known to mediate intercellular exchanging genetic information via secreted miRNAs from various cells. Therefore, extracellular miRNAs have attracted a major interest for their application as diagnostic biomarkers to detect early stages of cancers. Recently, we have been focusing on the structural variants of miRNAs, which have 1 or 2 base differences at the 3’-end of mature miRNAs, for their biological significance in cancer development. A specific structural variant of oncogenic miR-21 was found to be remarkably up-regulated in the lung cancer cell lines and clinical samples. Surprisingly, dominance of miR-21, a structural variant, is significantly associated with the recurrence of early stages of lung adenocarcinoma (LADC) patients. Mutation of the genes, encoding cell cycle regulators, and DNA-damage response were significantly enriched in the high variant dominant samples. Furthermore, cell cycle promotion, epithelial-mesenchymal transition, and the evasion of immune system were found to be major characteristics of the dominance tumor. These phenotypes are suggested to be induced by the inhibition of mature miR-21 function by the expression of structural variants (Figure 1).
2. Regulation of tumor-microenvironment (TME) by extracellular miRNA
Adaptation of sarcoma cells to their specific tumor microenvironment (TME) is a crucial component of the maintenance of their malignant properties. Intracellular communication is generally mediated by cell-cell contact or secreted proteins, including cytokines and chemokine. Accumulating evidence demonstrates that extracellular vesicles (EVs), including those in exosomes and other particles, released by tumor cells play a major role in the adaptation of malignant cells to TME. Extracellular microRNAs (exmiRNAs) embedded in the EVs have been reported to be involved in this adaptation, and also in the initiation of metastasis. Our extracellular miR-451a study has identified CMTM6 as a cellular target of miR-451a and its expression was demonstrated to be regulated by extracellular glucose level. To clarify the function, intracellular interacting molecules with CMTM6 were screened by immunoprecipitation-mass analysis. CMTM6 was found to form a complex with ER-resident intracellular molecular trafficking protein. CMTM6 knockdown (KD) has induced the downregulation of extracellular levels of specific secreting proteins, including MMPs. Furthermore, CMTM6 was also interacting with miRNA regulators, and its KD also affects extracellular levels of a certain subset of miRNAs. Our data indicated that CMTM6 has a crucial role in intracellular trafficking and secretion of secretory proteins and miRNAs.
3. Understanding the cellular networks specifically formed in p53-inactivated cancer cells
The tumor-suppressor gene, TP53, is mutated and/or inactivated in half of the human cancers, and dysfunction of p53 protein, a gene product of TP53, makes a critical contribution to the onset of carcinogenesis. Molecular network(s) specifically activated in p53-deficient contexts may promote the proliferation and survival of cancer cells. Therefore, understanding molecular bases for the activation of these networks showed the weaknesses of p53-deficient cancer cells, leading to the development of an ideal strategy for cancer therapy. We previously identified NEK9 as a regulator of the cell cycle in p53-deficient cells. NEK9 depression inhibited cell proliferation only in p53-deficient cells in vitro and in vivo. Parallel analyses have identified cellular networks activated in the p53 mutant context. Lung adenocarcinoma cases with p53 mutation showed significant up-regulation of this cellular network, suggesting this network-dependent proliferation in p53 mutant cancer cells. Detailed molecular cross-talks of NEK9 and this cellular network have been extensively analyzed.
Education
Supervising research and presentation skills for graduate school students and young scientists.
Future Prospects
Our studies aim to clarify cellular network(s) formed in cancer cells. Results obtained from these studies will provide molecular insights into how cancer cells were developed and progressed. Especially, research focusing on extracellular miRNA and biological roles of the structural variants of miRNAs is expected to find novel molecular mechanisms for cellular miRNA functions and intercellular communication between cancer cells and surrounding non-tumor cells, and these findings will be a new principle for the development of the next cancer therapeutics and diagnostics.