Annual Report 2020
Department of Omics Network
Masaru Katoh
Knowledge-Base Project
Masaru Katoh had worked in the fields of clinical medicine, molecular biology and genome science, and has been working in the field of precision medicine since 2015. Katoh concentrated the resources of the Department of Omics Network on its world-class major projects, such as FGF signaling cascades, Forkhead-box (FOX) family of transcription factors, Notch signaling cascades and WNT signaling cascades (http://orcid.org/0000-0003-3274-4066).
Precision medicine is defined as a medical system that utilizes clinical records, diagnostic imaging, laboratory tests, omics data and wearable device data for the prevention and treatment of human diseases. Artificial intelligence (AI) or machine learning technologies are applied for target discovery and drug screening in preclinical studies as well as diagnostic medical devices in clinical practices of cardiology, endocrinology, gastroenterology, neurology, oncology, ophthalmology, pathology and radiology. However, because the risk of bias is a critical issue for black box-type artificial intelligence, the human intelligence of multidisciplinary experts should be applied for improving the accuracy, robustness and stability of AI-based predictions.
In 2020, Masaru Katoh completed the Knowledge-Base Project to promote precision medicine for patients with cancers, cardiovascular diseases and neurodegenerative diseases. Katoh has published cutting-edge manuscripts on the Knowledge Base of FGF, Notch and WNT signaling networks as the corresponding author, including six “Highly Cited Papers” (Top 1%) in the Web of Science database (PMIDs: 23022474, 23696246, 27245147, 29048660, 30367139 and 31894255).
WNT signaling-targeted therapeutics
Whole-exome and whole-genome sequencing analyses on bulk tumors have revealed the genomic landscape of human cancers, including point mutations, fusions, gene amplifications and (super)enhancer alterations in cancer-related genes as well as numerous variants of unknown clinical significance. Diagnostic genome sequencing based on a panel of approximately 500 cancer-related genes has been applied in the clinic to identify targetable cancer drivers. Several cancer drivers are targeted by small-molecule inhibitors or antibody-based biologics in the clinic; however, there remain many cancer drivers that have not yet been successfully targeted in clinical practice.
For example, WNT signals are transduced to canonical and non-canonical pathways, and the canonical WNT signaling cascade is aberrantly activated in cancer patients owing to loss-of-function alterations in the APC, AXIN1, AXIN2, RNF43 and ZNRF3 genes or gain-of-function alterations in the CTNNB1 gene encoding β-catenin. Investigational WNT signaling blockers of diverse therapeutic modalities, such as small-molecule compounds, peptide mimetics, antibody-based drugs and CAR-T cells, have shown striking benefits in preclinical studies but have not yet been approved for the treatment of cancer patients.
Because the canonical WNT signaling cascade is involved in tumorigenesis as well as gastrointestinal, osteogenic and neuronal homeostasis, the therapeutic range of WNT signaling blockers might be too narrow for clinical application. Oncodevelopment signaling pathways with versatile functions in adult tissue homeostasis are hard and challenging targets for anticancer drug development.
Global Network Project
Preclinical studies using patient-derived cell lines, organoids and xenograft as well as animal models, such as monkey, mouse, Xenopus and zebrafish, are driving apparatuses for mechanistic understanding, target discovery and therapeutic optimization, while clinical trials are safety apparatuses for investigating the benefits and adverse effects of investigational diagnostics and therapeutics. Preclinical studies, clinical trials and medical practice are three essential components for the promotion of cyclical medical innovation (Figure 1).
Figure 1. Cyclical medical innovation for precision medicine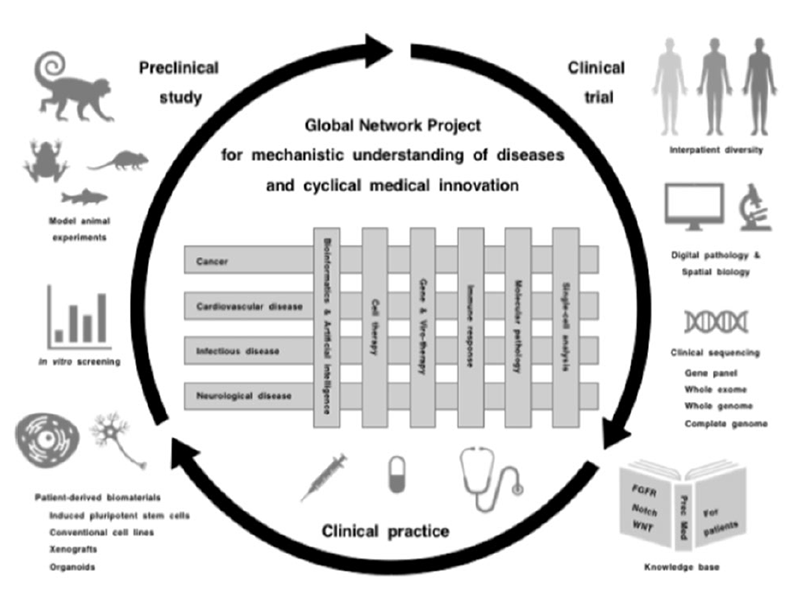

However, there are three major obstacles that hinder cyclical medical innovation. Basic studies are not always recapitulated in clinical trials due to intrinsic biases of cell lines, engineered mouse models and human organoids; investigational drugs are not always approved for the treatment of patients owing to unknown on-target adverse effects; and approved drugs are not always beneficial for patients even after selection using companion diagnostics.
Mechanistic understanding of human diseases is mandatory for developing novel diagnostics and therapeutics, and integrative interactions of scholars in the fields of basic, translational and clinical medicine as well as those of cancerous and noncancerous diseases are essential to promoting innovation cycles. Katoh is currently implementing the Global Network Project to provide a platform of knowledge generation and horizontal innovation for the promotion of cyclical medical innovation and improvement of human healthcare in the post-coronavirus era.
Contribution to the global scientific community
The manuscript citation count in the Web of Science Database is a surrogate marker for the contribution to the global scientific community. Masaru Katoh is the corresponding author of the six “Highly Cited Papers” in the Web of Science Database (http://orcid.org/0000-0003-3274-4066), and Katoh’s manuscripts were cited approximately 800 times by others in 2020.
