Annual Report 2021
Division of Cancer Immunology
Hiroyoshi Nishikawa, Shohei Koyama, Kosuke Tanaka, Takuma Irie, Kota Itahashi, Yasuko Tada, Rika Fujii, Sho Watanabe, Genki Okumura, Shogo Kumagai, Akito Kawazoe, Hirohiko Yamauchi, Junichiro Yuda, Kotaro Nomura, Yi-tzu Lin, Konomi Onagawa, Chika Takaku, Megumi Takemura, Megumi Hoshino, Mizuho Kikuchi, Haruko Ohira, Sayuri Yoshimatsu, Sho Isoyama, Tamiyo Kobayashi, Rieko Doi, Atsuo Sai, Daisuke Ito, Takuya Owari, Go Watanabe
Introduction
Cancer immunotherapy, represented by immune checkpoint inhibitors (ICIs), has become one of the standard treatment options for various cancers and is commonly administered in combination with conventional drugs. Various biomarkers such as PD-L1 expression and tumor mutation burden (TMB) have been reported as biomarkers to predict therapeutic response, and their usefulness has been demonstrated in certain cancers. However, markers based on stratification by immuno-genomic analysis of tumor tissues that can accurately predict therapeutic efficacy remain poorly understood. In addition, with the expansion of ICI indications, the number of cases of long-term response and relapse after long-term treatment is increasing, and the reasons for long-term response and the mechanisms by which resistance is acquired after long-term response also need to be clarified.
The Team and What We Do
To address these issues, our laboratory is collaborating with the clinical teams to search for novel biomarkers related to long-term response to ICIs, to elucidate the mechanisms involved in treatment resistance, and to propose combination treatment strategies targeting these mechanisms.
Focusing on regulatory T cells (Treg), which play a major role in suppressing anti-tumor immunity at the tumor site, we analyze biopsied tissue, blood, and stool samples before and after various treatments including ICIs, and comprehensively analyze cancer cells and immune cells by flow cytometry, multiple immunostaining, CyTOF, RNAseq, ATACseq, whole exome analysis, and metagenomic analysis (Figure 1). In addition, we are verifying the mechanisms of events observed in human specimens using mouse models. We are also actively involved in collaborative research with pharmaceutical companies, aiming to introduce new biomarkers into clinical trials and develop new immunotherapies and combination therapies.
Figure 1. We are investigating the dynamic immune state in
cancer patients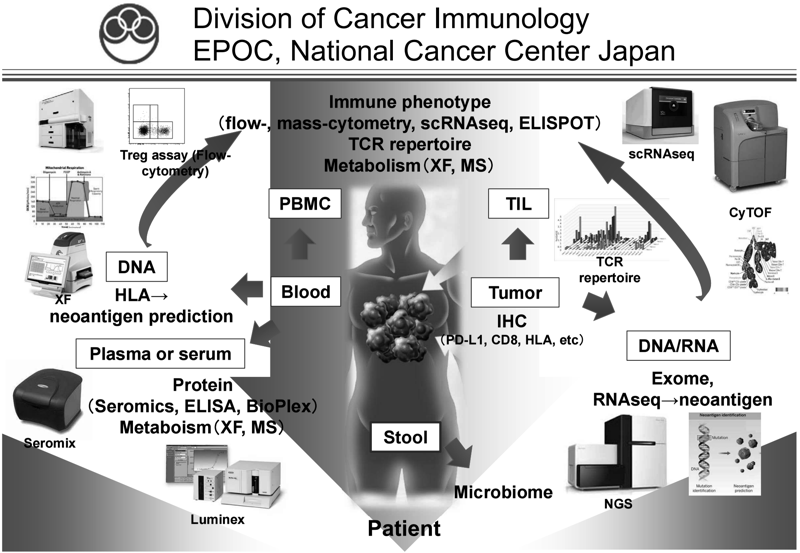

Research activities
As a prediction of therapeutic response to anti-PD-1 antibodies, our laboratory, in collaboration with a clinical team led by the Department of Gastroenterology and Respiratory Medicine of our hospital, has shown that the balance of PD-1 expression between CD8+ T cells and Tregs is crucial to distinguishing between response and non-response cases based on analysis of tumor-infiltrating T cells (Kumagai S et al., Nat Immunol 2020). However, the factors that regulate PD-1 expression in Tregs were unknown. Detailed analysis revealed that in tumors where the glycolysis is activated (e.g., MYC amplification and metastatic liver tumors), lactate production is enhanced and can be utilized as an energy source for Tregs but not for CD8+ T cells, which induces PD-1 expression in Tregs as compared to in CD8+ T cells (Figure 2) (Kumagai S et al., Cancer Cell 2022).
Figure 2. Developing immuno-precision biomarkers and Elucidating
the mechanisms of PD-1 expression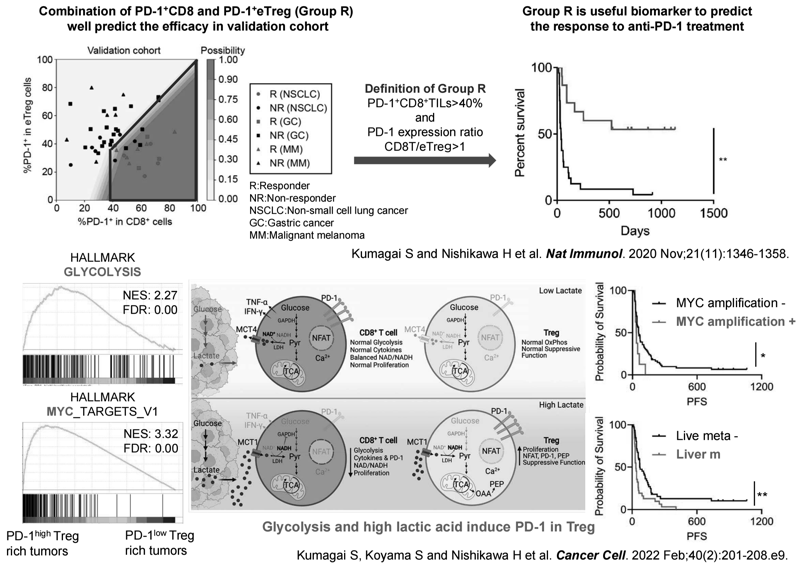

Furthermore, it was known that PD-L1 expression is decreased in some patients with non-small cell lung cancer (NSCLC) who have very high TMB and show ICI resistance. In collaboration with the Department of Respiratory Medicine and Thoracic Surgery of our hospital and Osaka University, we found the background mechanism of this phenotype in NSCLC. The WNT/β-catenin signaling-related genes appeared enriched in this subset of NSCLC. Interestingly, antigen-specific T cells were detected in peripheral blood but not in tumors, suggesting WNT/β-catenin signaling inhibits the infiltration of T cells (Figure. 3). A WNT inhibitor recovered T cell infiltration into tumors, which improved the efficacy of ICIs in murine models (Takeuchi Y et al. Sci Immunol 2021).
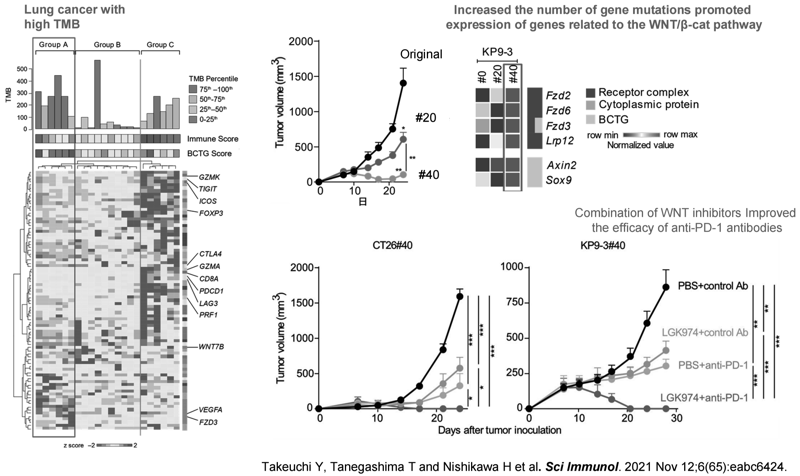
Clinical trials
We have collaborated with several pharmaceutical companies in their clinical trials to elucidate biomarkers for predicting the therapeutic response and to evaluate the immune status in the tumor microenvironment using our real-time immune monitoring.
Education
Many graduate school students are trained in our division, and young residents in National Cancer Center East are also trained to become physician scientists. After finishing their PhD course, some of students continue to study cancer immunology abroad.
Future Prospects
ICIs have undoubtedly opened a new era in cancer treatment. However, we still have several issues, including acquired resistance and suppressive immune modulation by somatic mutations in cancer cells, to overcome the drawbacks of ICIs. In order to answer these questions, immunological analyses using clinical specimens are essential. Therefore, it is important to develop better therapeutic strategies combining ICIs with other anti-cancer drugs and to identify biomarkers which can stratify responders from non-responders via clarifying the immune suppressive networks in the TME.
List of papers published in 2021
Journal
1. Aokage K, Tsuboi M, Zenke Y, Horinouchi H, Nakamura N, Ishikura S, Nishikawa H, Kumagai S, Koyama S, Kanato K, Kataoka T, Wakabayashi M, Fukutani M, Fukuda H, Ohe Y, Watanabe SI. Study protocol for JCOG1807C (DEEP OCEAN): a interventional prospective trial to evaluate the efficacy and safety of durvalumab before and after operation or durvalumab as maintenance therapy after chemoradiotherapy against superior sulcus non-small cell lung cancer. Japanese journal of clinical oncology, 52:383-387, 2022
2. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, Kamada T, Irie T, Okumura G, Kono H, Ito D, Fujii R, Watanabe S, Sai A, Fukuoka S, Sugiyama E, Watanabe G, Owari T, Nishinakamura H, Sugiyama D, Maeda Y, Kawazoe A, Yukami H, Chida K, Ohara Y, Yoshida T, Shinno Y, Takeyasu Y, Shirasawa M, Nakama K, Aokage K, Suzuki J, Ishii G, Kuwata T, Sakamoto N, Kawazu M, Ueno T, Mori T, Yamazaki N, Tsuboi M, Yatabe Y, Kinoshita T, Doi T, Shitara K, Mano H, Nishikawa H. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer cell, 40:201-218.e9, 2022
3. Hasegawa H, Shitara K, Takiguchi S, Takiguchi N, Ito S, Kochi M, Horinouchi H, Kinoshita T, Yoshikawa T, Muro K, Nishikawa H, Suna H, Kodera Y. A multicenter, open-label, single-arm phase I trial of neoadjuvant nivolumab monotherapy for resectable gastric cancer. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 25:619-628, 2022
4. Bando H, Tsukada Y, Inamori K, Togashi Y, Koyama S, Kotani D, Fukuoka S, Yuki S, Komatsu Y, Homma S, Taketomi A, Uemura M, Kato T, Fukui M, Wakabayashi M, Nakamura N, Kojima M, Kawachi H, Kirsch R, Yoshida T, Suzuki Y, Sato A, Nishikawa H, Ito M, Yoshino T. Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability-High Locally Advanced Rectal Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research, 28:1136-1146, 2022
5. Kawazu M, Ueno T, Saeki K, Sax N, Togashi Y, Kanaseki T, Chida K, Kishigami F, Sato K, Kojima S, Otsuka M, Kawazoe A, Nishinakamura H, Yuka M, Yamamoto Y, Yamashita K, Inoue S, Tanegashima T, Matsubara D, Tane K, Tanaka Y, Iinuma H, Hashiguchi Y, Hazama S, Khor SS, Tokunaga K, Tsuboi M, Niki T, Eto M, Shitara K, Torigoe T, Ishihara S, Aburatani H, Haeno H, Nishikawa H, Mano H. HLA Class I Analysis Provides Insight Into the Genetic and Epigenetic Background of Immune Evasion in Colorectal Cancer With High Microsatellite Instability. Gastroenterology, 162:799-812, 2022
6. Nagasaki J, Inozume T, Sax N, Ariyasu R, Ishikawa M, Yamashita K, Kawazu M, Ueno T, Irie T, Tanji E, Morinaga T, Honobe A, Ohnuma T, Yoshino M, Iwata T, Kawase K, Sasaki K, Hanazawa T, Kochin V, Kawamura T, Matsue H, Hino M, Mano H, Suzuki Y, Nishikawa H, Togashi Y. PD-1 blockade therapy promotes infiltration of tumor-attacking exhausted T cell clonotypes. Cell reports, 38:110331, 2022
7. Miyai Y, Sugiyama D, Hase T, Asai N, Taki T, Nishida K, Fukui T, Chen-Yoshikawa TF, Kobayashi H, Mii S, Shiraki Y, Hasegawa Y, Nishikawa H, Ando Y, Takahashi M, Enomoto A. Meflin-positive cancer-associated fibroblasts enhance tumor response to immune checkpoint blockade. Life science alliance, 5:2022
8. Kawazoe A, Itahashi K, Yamamoto N, Kotani D, Kuboki Y, Taniguchi H, Harano K, Naito Y, Suzuki M, Fukutani M, Higuchi T, Ikeno T, Wakabayashi M, Sato A, Koyama S, Nishikawa H, Shitara K. TAS-116 (Pimitespib), an Oral HSP90 Inhibitor, in Combination with Nivolumab in Patients with Colorectal Cancer and Other Solid Tumors: An Open-Label, Dose-Finding, and Expansion Phase Ib Trial (EPOC1704). Clinical cancer research: an official journal of the American Association for Cancer Research, 27:6709-6715, 2021
9. Izumi H, Matsumoto S, Liu J, Tanaka K, Mori S, Hayashi K, Kumagai S, Shibata Y, Hayashida T, Watanabe K, Fukuhara T, Ikeda T, Yoh K, Kato T, Nishino K, Nakamura A, Nakachi I, Kuyama S, Furuya N, Sakakibara-Konishi J, Okamoto I, Taima K, Ebi N, Daga H, Yamasaki A, Kodani M, Udagawa H, Kirita K, Zenke Y, Nosaki K, Sugiyama E, Sakai T, Nakai T, Ishii G, Niho S, Ohtsu A, Kobayashi SS, Goto K. The CLIP1-LTK fusion is an oncogenic driver in non-small-cell lung cancer. Nature, 600:319-323, 2021
10. Kashima Y, Shibahara D, Suzuki A, Muto K, Kobayashi IS, Plotnick D, Udagawa H, Izumi H, Shibata Y, Tanaka K, Fujii M, Ohashi A, Seki M, Goto K, Tsuchihara K, Suzuki Y, Kobayashi SS. Single-Cell Analyses Reveal Diverse Mechanisms of Resistance to EGFR Tyrosine Kinase Inhibitors in Lung Cancer. Cancer research, 81:4835-4848, 2021
11. Isoyama S, Mori S, Sugiyama D, Kojima Y, Tada Y, Shitara K, Hinohara K, Dan S, Nishikawa H. Cancer immunotherapy with PI3K and PD-1 dual-blockade via optimal modulation of T cell activation signal. Journal for immunotherapy of cancer, 9:2021
12. Maeda Y, Wada H, Sugiyama D, Saito T, Irie T, Itahashi K, Minoura K, Suzuki S, Kojima T, Kakimi K, Nakajima J, Funakoshi T, Iida S, Oka M, Shimamura T, Doi T, Doki Y, Nakayama E, Ueda R, Nishikawa H. Depletion of central memory CD8+ T cells might impede the antitumor therapeutic effect of Mogamulizumab. Nature communications, 12:7280, 2021
13. Fujioka Y, Sugiyama D, Matsumura I, Minami Y, Miura M, Atsuta Y, Ohtake S, Kiyoi H, Miyazaki Y, Nishikawa H, Takahashi N. Regulatory T Cell as a Biomarker of Treatment-Free Remission in Patients with Chronic Myeloid Leukemia. Cancers, 13:2021
14. Takeuchi Y, Tanegashima T, Sato E, Irie T, Sai A, Itahashi K, Kumagai S, Tada Y, Togashi Y, Koyama S, Akbay EA, Karasaki T, Kataoka K, Funaki S, Shintani Y, Nagatomo I, Kida H, Ishii G, Miyoshi T, Aokage K, Kakimi K, Ogawa S, Okumura M, Eto M, Kumanogoh A, Tsuboi M, Nishikawa H. Highly immunogenic cancer cells require activation of the WNT pathway for immunological escape. Science immunology, 6:eabc6424, 2021
15. Kawashima S, Inozume T, Kawazu M, Ueno T, Nagasaki J, Tanji E, Honobe A, Ohnuma T, Kawamura T, Umeda Y, Nakamura Y, Kawasaki T, Kiniwa Y, Yamasaki O, Fukushima S, Ikehara Y, Mano H, Suzuki Y, Nishikawa H, Matsue H, Togashi Y. TIGIT/CD155 axis mediates resistance to immunotherapy in patients with melanoma with the inflamed tumor microenvironment. Journal for immunotherapy of cancer, 9:2021
16. Watanabe K, Nishikawa H. Engineering strategies for broad application of TCR-T- and CAR-T-cell therapies. International immunology, 33:551-562, 2021
17. Nishikawa H, Koyama S. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. Journal for immunotherapy of cancer, 9:2021
18. Satoh K, Kobayashi Y, Fujimaki K, Hayashi S, Ishida S, Sugiyama D, Sato T, Lim K, Miyamoto M, Kozuma S, Kadokura M, Wakita K, Hata M, Hirahara K, Amano M, Watanabe I, Okamoto A, Tuettenberg A, Jonuleit H, Tanemura A, Maruyama S, Agatsuma T, Wada T, Nishikawa H. Novel anti-GARP antibody DS-1055a augments anti-tumor immunity by depleting highly suppressive GARP+ regulatory T cells. International immunology, 33:435-446, 2021
19. Yasuda Y, Iwama S, Sugiyama D, Okuji T, Kobayashi T, Ito M, Okada N, Enomoto A, Ito S, Yan Y, Sugiyama M, Onoue T, Tsunekawa T, Ito Y, Takagi H, Hagiwara D, Goto M, Suga H, Banno R, Takahashi M, Nishikawa H, Arima H. CD4+ T cells are essential for the development of destructive thyroiditis induced by anti-PD-1 antibody in thyroglobulin-immunized mice. Science translational medicine, 13:2021
20. Kobayashi T, Iwama S, Sugiyama D, Yasuda Y, Okuji T, Ito M, Ito S, Sugiyama M, Onoue T, Takagi H, Hagiwara D, Ito Y, Suga H, Banno R, Nishikawa H, Arima H. Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. Journal for immunotherapy of cancer, 9:2021
21. Kitahara M, Shigeno Y, Shime M, Matsumoto Y, Nakamura S, Motooka D, Fukuoka S, Nishikawa H, Benno Y. Vescimonas gen. nov., Vescimonas coprocola sp. nov., Vescimonas fastidiosa sp. nov., Pusillimonas gen. nov. and Pusillimonas faecalis sp. nov. isolated from human faeces. International journal of systematic and evolutionary microbiology, 71:2021
22. Kelkka T, Savola P, Bhattacharya D, Huuhtanen J, Lönnberg T, Kankainen M, Paalanen K, Tyster M, Lönnberg M, Ellonen P, Smolander J, Eldfors S, Yadav B, Khan S, Koivuniemi R, Sjöwall C, Elo LL, Lähdesmäki H, Maeda Y, Nishikawa H, Leirisalo-Repo M, Sokka-Isler T, Mustjoki S . Corrigendum: Adult-Onset Anti-Citrullinated Peptide Antibody-Negative Destructive Rheumatoid Arthritis Is Characterized by a Disease-Specific CD8+ T Lymphocyte Signature. Frontiers in immunology, 12:710831, 2021
23. Saito T, Kurose K, Kojima T, Funakoshi T, Sato E, Nishikawa H, Nakajima J, Seto Y, Kakimi K, Iida S, Doki Y, Oka M, Ueda R, Wada H. Phase Ib study on the humanized anti-CCR4 antibody, KW-0761, in advanced solid tumors. Nagoya journal of medical science, 83:827-840, 2021
24. Tanaka K, Yu HA, Yang S, Han S, Selcuklu SD, Kim K, Ramani S, Ganesan YT, Moyer A, Sinha S, Xie Y, Ishizawa K, Osmanbeyoglu HU, Lyu Y, Roper N, Guha U, Rudin CM, Kris MG, Hsieh JJ, Cheng EH. Targeting Aurora B kinase prevents and overcomes resistance to EGFR inhibitors in lung cancer by enhancing BIM- and PUMA-mediated apoptosis. Cancer cell, 39:1245-1261.e6, 2021
