Annual Report 2021
Data Science Strategy Section
Takashi Kohno, Katsuya Tsuchihara, Genta Ohno, Haruka Nakada, Hirokazu Fukuda
The Team and What We Do
The mission of this section is to construct various data-sharing systems of the C-CAT repository data consisting of clinical and genomic data of cancer patients who received comprehensive genomic profiling (CGP) tests (Fig.1). Since September 2020, the C-CAT data have been shared by physicians and medical staff within the cancer genomic medicine (CGM) hospitals through the “C-CAT Medical-Use Portal”. In addition, we offer a data-sharing system, the “C-CAT Research-Use Portal”, through which researchers in academic institutions and industries can access the repository data (clinical information and mutation lists) under the approval of the C-CAT Data Utilization Review Board. To extend our data sharing, we are building an additional cloud-based system, “C-CAT CALICO (CALculation & Investigation ClOud)”, which enables users to analyze raw sequence data to examine novel genetic alterations that are not included in the reports provided by the testing companies.
We also maintain operational aspects of these data-sharing systems, including publicity, consultations, acceptance of applications and contracts with users, as well as holding the C-CAT Data Utilization Review Board. Furthermore, we do public relations activities about CGM and CGP tests for patients and the general public.
Figure 1. Medical and Research Use of C-CAT “Real
World” Data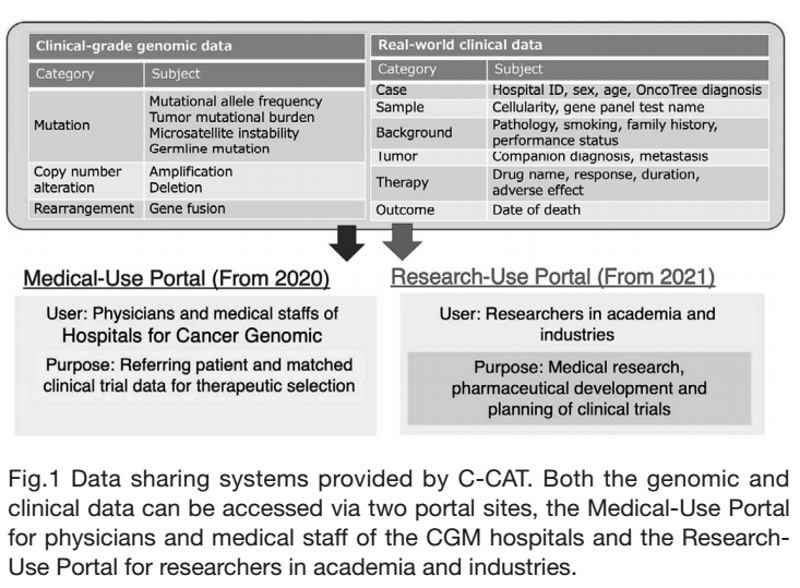

Activities
The “C-CAT Research-Use Portal” was launched in October 2021. Encouragingly, as of March 2022, 99.7% of patients gave consent to share their data with the third parties, and therefore, nearly all C-CAT data can be accessed by nonacademic institutions in addition to academia under the approval of the C-CAT Data Utilization Review Board. Twenty-four academic and industrial institutions in Japan (including Japanese branches of foreign pharmaceutical companies) have been permitted to use the C-CAT Research-Use Portal by the C-CAT Data Utilization Review Board as of the end of March 2022.
Both the C-CAT Medical-Use and the C-CAT Research-Use Portal systems were continuously renovated to improve usability. In order to provide information on CGM and CGP tests for patients and the general public, we joined Twitter and published newsletters as well as enhancing the website.
Future prospects
The C-CAT Research-Use Portal will become open to the broad research community outside our nation. The C-CAT CALICO will also been launched shortly. We hope that these data-sharing systems will play a significant role in expanding/advancing cancer treatments and improving cancer care worldwide.
